Key Message
Nirmatrelvir and ritonavir are two co-administered antiviral medications, marketed under the name Paxlovid in Canada, for the treatment of SARS-CoV-2 infection. Nirmatrelvir is an inhibitor of SARS-CoV-2 3CL-like protease that prevents polyprotein cleavage of proteins necessary for SARS-CoV-2 genome replication. Nirmatrelvir has been studied in combination with ritonavir, a medication that has no known activity against SARS-CoV-2 but slows the metabolism of nirmatrelvir by inhibiting hepatic enzymes, thus “boosting” concentrations of nirmatrelvir.
Nirmatrelvir/ritonavir is currently approved in Canada for the treatment of mild COVID-19 (termed “mild to moderate” with Health Canada’s terminology) in those who are at high risk for progression to severe or critical COVID-19 illness. It is not approved for the treatment of patients requiring hospitalization due to COVID-19, nor for pre- or post-exposure prophylaxis for the prevention of SARS-CoV-2 infection. As of the date of publication of this Science Brief, there is one peer-reviewed publication comparing nirmatrelvir/ritonavir against placebo.
Nirmatrelvir/ritonavir reduces the incidence of hospitalization and/or death in patients with mild COVID-19 with risk factors for progression to moderate or critical illness, with fewer treatment-emergent serious adverse events relative to placebo.
The panel noted a marginal benefit in individuals at low risk of hospitalization, and the high certainty of harm with nirmatrelvir/ritonavir if known drug-drug interactions are not mitigated.
Critically Ill Patients (On High-Flow Oxygen, Mechanical Ventilation, or Extracorporeal Membrane Oxygenation (ECMO))
Nirmatrelvir/ritonavir is not recommended for critically ill patients with COVID-19.
Moderately Ill Patients (On Low-Flow Oxygen)
Nirmatrelvir/ritonavir is not recommended for moderately ill patients with COVID-19.
Mildly Ill Patients (Not Requiring Supplemental Oxygen)
Nirmatrelvir/ritonavir is recommended at a dose of 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet), with all three tablets taken together orally twice daily for five days. Patients must be at higher risk of severe disease from COVID-19 and present within five days of symptom onset.
Risk factors for progression to moderate or critical COVID-19 are outlined in “Methods used for this Science Brief” below, and include immunocompromised individuals, and individuals whose combination of age, vaccination history, and risk factors put them at increased risk of progression to severe disease.
Other treatment options that may be available to these higher risk patients include sotrovimab, remdesivir, fluvoxamine, and budesonide. Clinicians should consider patient-specific factors including (but not limited to) drug-drug interactions, renal function, duration of COVID-19 symptoms, ability to administer intravenous versus oral drugs, strength of evidence, situational context, and drug supply in decision-making regarding choice of therapy (see Therapeutic Management of Adult Patients with COVID-19 summary for additional details).
If eligible patients with mild COVID-19 who began treatment with nirmatrelvir/ritonavir progress to moderate COVID-19 during their treatment course, they may complete their treatment course at the discretion of the treating physician.
Implementation Considerations
It is recommended that oral antiviral therapy be administered to non-hospitalized individuals across Ontario using a hybrid network that includes, but is not limited to, mobile integrated healthcare services, community paramedicine, virtual/remote assessment, and outpatient clinics.
Special Populations
Pharmacist consultation is important to mitigate any significant drug-drug interactions (including natural products). Nirmatrelvir/ritonavir is contraindicated in patients taking certain medications that have the potential for serious or life-threatening reactions at high concentrations and are highly dependent on CYP3A4-mediated metabolism; and in patients taking certain medications that are CYP3A-inducing, as these may significantly decrease concentrations of nirmatrelvir/ritonavir, decreasing its efficacy as a COVID-19 treatment (see Tables 1-4 in the “Considerations” section below, and the guidance document “Nirmatrelvir/ritonavir (Paxlovid): What prescribers and pharmacists need to know”). For complex interactions, consultation with a pharmacist experienced in managing ritonavir-related interactions may be helpful.
In patients with moderate renal impairment (eGFR ≥30 to <60 mL/min), the dose should be reduced to 150 mg nirmatrelvir (one 150 mg tablet) and 100 mg ritonavir (one 100 mg tablet) taken together twice daily for 5 days. Nirmatrelvir/ritonavir is not recommended in patients with severe renal impairment (eGFR <30 mL/min) or who require dialysis.
Nirmatrelvir/ritonavir may be considered for the treatment of pregnant patients with mild COVID-19 who otherwise meet the criteria outlined for mildly ill patients. There is a lack of data on nirmatrelvir/ritonavir use in pregnant patients; however, there is extensive experience with ritonavir use in pregnant patients living with HIV. If a pregnant patient fits the risk profile to potentially derive benefit from nirmatrelvir/ritonavir, the risks and benefits of initiating treatment should be discussed with the patient. Care of pregnant patients with COVID-19 should be managed by a multidisciplinary team with suitable expertise in the management of pregnancy.
Nirmatrelvir/ritonavir may be considered for lactating patients with mild COVID-19 who otherwise meet the criteria outlined for mildly ill patients. There is a lack of data on nirmatrelvir/ritonavir use in lactating patients; however, based on studies in HIV, for which ritonavir is also used, it is known that ritonavir may be present in breast milk. If a lactating patient fits the risk profile to potentially derive benefit from nirmatrelvir/ritonavir, the risks and benefits of initiating treatment should be discussed with the patient. We recommend advising the patient not to breastfeed for the duration of treatment and four days afterwards, during which time breast milk should be pumped and discarded.
Lay Summary
When the virus that causes COVID-19, SARS-CoV-2, infects you, it enters the cells in your throat and lungs. Once inside your cells, the virus makes copies of itself, releasing more viruses which can then infect more cells. It does this by making copies of its genetic code which it uses to assemble new virus. Nirmatrelvir/ritonavir (NEER-mah-TREL-veer/ree-TOW-nah-VEER), are two drugs marketed as Paxlovid that, when taken together, stop this process.
Nirmatrelvir/ritonavir is licensed by Health Canada to treat adult (>18 years of age) patients with COVID-19 who are well enough to be cared for at home but have a higher chance of becoming very sick from COVID-19 and needing to be cared for in hospital.
How Paxlovid (Nirmatrelvir/Ritonavir) Works
Paxlovid is the trade name of nirmatrelvir and ritonavir which are two drugs taken by mouth that come packaged together in a blister pack. Nirmatrelvir works by interfering with processing proteins that SARS-CoV-2 needs to be able to make more copies of itself. Ritonavir does not have any effect on SARS-CoV-2 but increases how long nirmatrelvir can work in your body by slowing how the liver breaks it down. Without ritonavir, it would be difficult for your body to have enough nirmatrelvir in it to be able to stop the virus from making copies of itself.
How We Came to Our Recommendations
One peer-reviewed study has been published on nirmatrelvir/ritonavir. Patients were given either nirmatrelvir and ritonavir (two nirmatrelvir pills and one ritonavir pill taken at the same time) or a placebo twice a day for five days along with usual care. A placebo is a pill that looks the same as nirmatrelvir and ritonavir, but contains no active ingredients. Neither the doctors nor patients knew whether the patients with COVID-19 received nirmatrelvir/ritonavir or placebo. The trial showed that in patients with COVID-19 who were well enough to be cared for at home, nirmatrelvir/ritonavir decreases the chance of hospitalization (in the study, hospitalization was defined as patients staying in the hospital, emergency department, or acute medical care unit of a nursing home for more than 24 hours).1 The data only looked at patients with a higher chance of becoming very sick from COVID-19. The patients in the study were rather young – the average age was 46 and roughly one out of every five participants were over age 60. Only unvaccinated patients without a previous SARS-CoV-2 infection could take part in the study, so they had no immunity to SARS-CoV-2. There were no concerns that patients receiving nirmatrelvir/ritonavir had more side effects than the ones receiving placebo. In another unpublished study in people at relatively standard risk of hospitalization (including being vaccinated but having at least one risk factor), nirmatrelvir/ritonavir did not help patients feel better faster. It is important to know whether oral medications that block SARS-CoV-2 from making more copies of itself work against the variants currently making people sick from COVID-19 in Ontario: in a preprint study that has been posted but not yet peer-reviewed, nirmatrelvir/ritonavir was shown to work against the Omicron variant, which is currently the most common variant in Ontario.2
Our Recommendations
We do not recommend nirmatrelvir/ritonavir for patients with COVID-19 who are already sick enough to need extra oxygen. These patients are usually cared for in hospital, and might benefit more from other treatments.
We recommend nirmatrelvir/ritonavir for patients with COVID-19 that have a higher chance of becoming moderately or severely ill but are still well enough to not need extra oxygen. Patients with a higher chance of becoming moderately or severely ill from COVID-19 include those who are elderly, are Indigenous, have immune system dysfunction or are on immune system-weakening drugs, or have other medical concerns including cancer, heart disease, lung disease, kidney disease, diabetes, or obesity. Patients who have less than 3 doses of COVID-19 vaccination also have a higher chance of becoming moderately or severely ill.3 Two nirmatrelvir pills and one ritonavir pill are taken morning and evening at the same time twice a day for five days, and can be taken with or without food.
We recommend that before a patient starts taking nirmatrelvir/ritonavir, they talk to a pharmacist experienced with managing medication interactions to make sure they are not on any medications, natural products, or supplements that can cause problems when you take nirmatrelvir/ritonavir. Because ritonavir works by stopping your liver from breaking down nirmatrelvir, it can also stop other drugs from being broken down by your liver. With some medications, this can lead to side effects that can be serious or cause death.
We do not recommend nirmatrelvir/ritonavir for patients with severe kidney or liver disease.
We recommend nirmatrelvir/ritonavir for patients with COVID-19 who have mild to moderate kidney disease and have a higher chance of becoming very sick but do not need extra oxygen. These patients should take a smaller dose: one nirmatrelvir pill and one ritonavir pill taken at the same time twice a day for five days, with or without food.
We recommend nirmatrelvir/ritonavir for patients with COVID-19 who have mild to moderate liver disease and have a higher chance of becoming very sick but do not need extra oxygen. These patients can take the normal dose: two nirmatrelvir pills and one ritonavir pill taken at the same time twice a day for five days, with or without food.
Patients living with HIV who are already taking ritonavir or cobicistat (another medication that works by slowing down how the liver breaks down other drugs) as part of their treatment should keep taking their regular doses of HIV medications without stopping or changing them while on nirmatrelvir/ritonavir. If a patient living with HIV is not on antiretroviral medication, we do not recommend taking nirmatrelvir/ritonavir because it might cause their HIV to become resistant to some antiretroviral medications.
Patients who are pregnant, are well enough to not need extra oxygen, and who have a higher chance of getting very sick from COVID-19 can consider taking nirmatrelvir/ritonavir after talking about the risks and benefits with their doctor, and have reviewed any medications they are on with a pharmacist who is experienced in dealing with medication interactions.
Patients with COVID-19 who are breastfeeding, are well enough to not need extra oxygen, and who have a higher chance of getting very sick from COVID-19 can consider taking nirmatrelvir/ritonavir after talking about the risks and benefits with their doctor, and have reviewed any medications they are on with a pharmacist who is experienced in dealing with medication interactions. If a breastfeeding patient starts nirmatrelvir/ritonavirtreatment, they should not breastfeed for nine days (five days while taking nirmatrelvir/ritonavir, and four days afterwards). During those nine days, breastfeeding patients should pump and dispose of any expressed breast milk.
Summary
Background
Nirmatrelvir/ritonavir (NEER-mah-TREL-veer/ree-TOW-nah-VEER), marketed as Paxlovid, is a combination oral therapy for the treatment of SARS-CoV-2 infection. Nirmatrelvir inhibits proteolysis by binding the 3CL protease, ultimately leading to the cessation of viral replication. Ritonavir, which is co-administered with nirmatrelvir, is not active against SARS-CoV-2 and acts as a “booster agent” by inhibiting CYP3A4, thereby maximizing the nirmatrelvir concentration in plasma.
Questions
Does nirmatrelvir/ritonavir improve patient outcomes including mortality, need for mechanical ventilator or organ support, hospitalization, and resolution of symptoms for individuals with COVID-19 who do not require supplemental oxygen therapy?
What is the serious adverse event profile of nirmatrelvir/ritonavir?
How should nirmatrelvir/ritonavir be used during the current Omicron wave?
Findings
Two large, multinational randomized, placebo-controlled trials were identified one in a population at high risk of poor outcomes, and one in a standard risk population.
In the peer-reviewed trial, EPIC-HR, study subjects (managed in the community but considered high risk for progression to severe disease with COVID-19) in whom we based our analysis received their first dose after randomization within five days of symptom onset and did not receive monoclonal antibodies for COVID-19. Of these patients, those who received nirmatrelvir/ritonavir (n=1,039) had reduced hospitalizations, mortality, and the composite outcome of hospitalizations or death, compared to those who received placebo (n=1,046). For the composite outcome of hospitalization or death, the risk ratio (RR) of 0.12 (95% confidence interval (CI)0.06 – 0.25) corresponds to a 5.5% absolute risk reduction, meaning one additional outcome is prevented for approximately every 18 patients treated. Young age and lack of details on concomitant medication use limit study generalizability. The impact of nirmatrelvir/ritonavir on the use of invasive mechanical ventilation (IMV) was not described.
Patients who received nirmatrelvir/ritonavir had fewer serious adverse events, and adverse events overall than those who received placebo – though the low proportion of enrolled patients ≥60 years of age may bias these conclusions. There are many known drug-drug interactions, particularly with ritonavir, and the inclusion criteria of the study may not be reflective of those who may derive the most benefit from this medication in Ontario due to the complexities of prescribing this medication.
In the unpublished, non-peer-reviewed EPIC-SR study, unvaccinated study subjects considered standard risk for severe disease progression with COVID-19, received their first dose within five days of symptom onset, and were not treated with monoclonal antibodies for COVID-19. Vaccinated people with 1 additional risk factor were also eligible for study entry. Results from this ongoing study are unavailable, but a press release reported that there was no appreciable difference between nirmatrelvir/ritonavir and placebo: with 662 subjects enrolled for interim analysis, 2/333 (0.6%) receiving nirmatrelvir/ritonavir and 8/329 (2.4%) receiving placebo were hospitalized.
Recommendations
Critically Ill Patients (On High-Flow Oxygen, Mechanical Ventilation, or Extracorporeal Membrane Oxygenation (ECMO))
Nirmatrelvir/ritonavir is not recommended for critically ill patients with COVID-19.
Moderately Ill Patients (On Low-Flow Oxygen)
Nirmatrelvir/ritonavir is not recommended for moderately ill patients with COVID-19.
Mildly Ill Patients (Not Requiring Supplemental Oxygen)
Nirmatrelvir/ritonavir is recommended at a dose of 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet), with all three tablets taken together orally twice daily for 5 days. Patients must be at a higher risk of severe disease from COVID-19 and present within 5 days of symptom onset.
Risk factors for progression to moderate or critical COVID-19 are outlined in in “Methods used for this Science Brief” below, and include immunocompromised individuals, and individuals whose combination of age, vaccination history, and risk factors put them at increased risk of progression to severe disease.
Other treatment options that may be available to these higher risk patients include sotrovimab, remdesivir, fluvoxamine, and budesonide. Clinicians should consider patient-specific factors including (but not limited to) drug-drug interactions, renal function, duration of COVID-19 symptoms, ability to administer intravenous versus oral drugs, strength of evidence, situational context, and drug supply in decision-making regarding choice of therapy (see Therapeutic Management of Adult Patients with COVID-19 summary for additional details).4
If eligible patients with mild COVID-19 who began treatment with nirmatrelvir/ritonavir progress to moderate COVID-19 during their treatment course, they may complete their treatment course at the discretion of the treating physician.
Implementation Considerations
It is recommended that oral antiviral therapy be administered to non-hospitalized individuals across Ontario using a hybrid network that includes, but is not limited to, mobile integrated healthcare services, community paramedicine, virtual/remote assessment, and outpatient clinics.
Special Populations
Pharmacist consultation is important to mitigate any significant drug-drug interactions (including natural products). Nirmatrelvir/ritonavir is contraindicated in patients taking certain medications that have the potential for serious or life-threatening reactions at high concentrations and are highly dependent on CYP3A4-mediated metabolism; and in patients taking certain medications that are CYP3A-inducing, as these may significantly decrease concentrations of nirmatrelvir/ritonavir, decreasing its efficacy as a COVID-19 treatment (see Tables 1-4 in the “Considerations” section below, and the guidance document “Nirmatrelvir/ritonavir (Paxlovid): What prescribers and pharmacists need to know”).5 For complex interactions, consultation with a pharmacist experienced in managing ritonavir-related interactions is recommended.
In patients with moderate renal impairment (eGFR ≥30 to <60 mL/min), the dose should be reduced to 150 mg nirmatrelvir (one 150 mg tablet) and 100 mg ritonavir (one 100 mg tablet) taken together twice daily for 5 days. Nirmatrelvir/ritonavir is not recommended in patients with severe renal impairment (eGFR <30 mL/min) or who require dialysis.
Nirmatrelvir/ritonavir may be considered for the treatment of pregnant patients with mild COVID-19 who otherwise meet the criteria outlined for mildly ill patients. There is a lack of data on nirmatrelvir/ritonavir use in pregnant patients; however, there is extensive experience with ritonavir use in pregnant patients living with HIV. If a pregnant patient fits the risk profile to potentially derive benefit from nirmatrelvir/ritonavir, the risks and benefits of initiating treatment should be discussed with the patient. Care of pregnant patients with COVID-19 should be managed by a multidisciplinary team with suitable expertise in the management of pregnancy.
Nirmatrelvir/ritonavir may be considered for lactating patients with mild COVID-19 who otherwise meet the criteria outlined for mildly ill patients. There is a lack of data on nirmatrelvir/ritonavir use in lactating patients; however, based on studies in HIV, for which ritonavir is also used, it is known that ritonavir may be present in breast milk. If a lactating patient fits the risk profile to potentially derive benefit from nirmatrelvir/ritonavir, the risks and benefits of initiating treatment should be discussed with the patient. We recommend advising the patient not to breastfeed for the duration of treatment and four days afterwards, during which time breast milk should be pumped and discarded.
Full Text
Background
COVID-19 is characterized by an initial replicative phase where viral replication is the predominant driver of illness, and an inflammatory phase during which host responses predominate. Treatments that inhibit viral replication, if administered early in the course of illness, may improve patient-important outcomes, particularly in those at increased risk of progression to moderate or critical illness. In the context of the Omicron variant, the optimal timing for treatment before the inflammatory phase of illness is unclear.
Paxlovid (nirmatrelvir/ritonavir) is a combination oral therapy for the treatment of SARS-CoV-2 infection. Nirmatrelvir is a selective inhibitor of the SARS-CoV-2 3CL-like protease (also known as main protease or 3CLpro).6 3CLpro is required for the processing of polyprotein precursors, which generates proteins necessary for SARS-CoV-2 replication and transcription. By inhibiting 3CLpro, nirmatrelvir inhibits proteolysis before viral RNA replication, leading to the cessation of downstream viral replication. Ritonavir, which is coadministered with nirmatrelvir, is not active against SARS-CoV-2. Ritonavir was initially developed as a protease inhibitor of HIV-1 and is currently licensed for this use. When administered at low doses, ritonavir primarily acts as a “boosting agent” for another drug that is metabolized by CYP3A4: in this case, nirmatrelvir. By inhibiting CYP3A4, ritonavir maximizes the nirmatrelvir concentration in plasma.
Nirmatrelvir/ritonavir has been assessed in multiple animal models and in vitro studies demonstrating its effectiveness against SARS-CoV-2. A study has shown that nirmatrelvir retains activity in vitro against the Alpha, Beta, Gamma, Delta, and Omicron variants of concern.7 There is one peer-reviewed randomized controlled trial (RCT) currently published, EPIC-HR, which examines the use of nirmatrelvir/ritonavir in patients with mild COVID-19 at high risk of progression to COVID-19-related critical illness.1 An additional study in patients at standard risk of progression to COVID-19-related critical illness was identified but remains incompletely reported and is not yet peer reviewed.
Nirmatrelvir/ritonavir has been granted full approval in Canada for use in patients with mild COVID-19 (as per the Drugs & Biologics Clinical Practice Guidelines Working Group’s definitions) at high risk of progression to moderate or severe illness.
Projected demand for oral treatments for SARS-CoV-2 infection, such as nirmatrelvir/ritonavir, is expected to be high, particularly among SARS-CoV-2-infected individuals who do not have immunity to SARS-CoV-2 because they are unvaccinated, have waned immunity, are immunocompromised (and thus lack an inadequate immune response to vaccination), or because of viral immune escape. Nirmatrelvir/ritonavir has the potential to significantly alleviate healthcare system burden as it can be administered to outpatients without the need for specialized equipment and personnel required for intravenous treatments.
Questions
Does nirmatrelvir/ritonavir improve patient outcomes including mortality, need for mechanical ventilator or organ support, hospitalization, and resolution of symptoms for individuals with COVID-19 who do not require supplemental oxygen therapy?
What is the serious adverse event profile of nirmatrelvir/ritonavir?
How should nirmatrelvir/ritonavir be used during the current Omicron wave?
Findings
As of February 16, 2022, we identified one published peer-reviewed study comparing nirmatrelvir/ritonavir with placebo for the treatment of COVID-19.1 This study, EPIC-HR, was completed in unvaccinated patients with increased risk for progression to COVID-19-related critical illness. Another RCT was identified but has yet to be fully reported and published as a peer-reviewed paper. This unpublished study (EPIC-SR, NCT05011513) looked at low-risk infected individuals, and interim data is only currently available via press release.
Nirmatrelvir/ritonavir is also being assessed in an ongoing RCT for post-exposure prophylaxis in household contacts of symptomatic patients with COVID-19 (NCT05047601).
Nirmatrelvir/Ritonavir vs. Placebo in Patients with Increased Risk for Progression to Severe Disease
EPIC-HR (NCT04960202) is a phase III double-blind, placebo-controlled RCT that compared nirmatrelvir/ritonavir versus placebo in unvaccinated, symptomatic, non-hospitalized patients with laboratory-confirmed SARS-CoV-2 infection within five days of first onset of symptoms who are at increased risk of progression to severe disease and received usual care. Findings were published on February 16, 2022.1
The study included patients ≥18 years old who had mild to moderate COVID-19 illness using United States Food and Drug Administration criteria. This illness severity corresponds to the Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group’s definition of mild COVID-19 illness (see “Methods Used for this Science Brief” below). Included individuals were not vaccinated against SARS-CoV-2, had symptomatic SARS-CoV-2 infection confirmed by laboratory testing, and were randomized within five days of symptom onset. Patients were required to have at least one symptom attributable to COVID-19 on the Day 1 evaluation, and at least one risk factor for progression to severe illness.
Risk factors were defined as: age ≥ 60 years; body mass index (BMI) >25 kg/m2; current smoker (within 30 days of randomization) or a history of at least 100 lifetime cigarettes; immunosuppressive disease (bone marrow transplantation or primary immunodeficiency) or prolonged use of immune-modulating medications (prednisone 20 mg equivalent for at least 14 consecutive days within 30 days preceding study entry; biologics, immunomodulators, or chemotherapy within 90 days of study entry; HIV with CD4 <200 cells/µL and viral load<400 copies/mL); chronic lung disease; known hypertension; cerebrovascular disease; diabetes mellitus, chronic kidney disease that does not meet exclusion criteria (below); sickle cell disease; neurodevelopmental disorders; genetic or metabolic syndromes; severe congenital abnormalities; active cancer; or medical-related technological dependence unrelated to COVID-19 (i.e., continuous positive airway pressure device).
The study excluded patients who were vaccinated against SARS-CoV-2 prior to the Day 34 visit, received convalescent plasma, had a history of prior SARS-CoV-2 infection or hospitalization for previous COVID-19, had a current or anticipated need for hospitalization within the 48 hours after study randomization, history of active liver disease, moderate-to-severe renal dysfunction, patients receiving dialysis, those with oxygen saturations at rest ≤92% within 24 hours of randomization, those with a comorbidity requiring hospitalization and/or surgery within seven days prior to study entry, or considered life-threatening within 30 days prior to study entry. Patients with a suspected or confirmed infection other than COVID-19, and patients with HIV-1 infection and a viral load >400 copies/mL were excluded. The study also excluded patients on medications that are highly dependent on CYP3A4 for clearance and for which elevated plasma concentrations may be associated with serious and/or life-threatening events, as well as medications that are strong inducers of CYP3A within 28 days of the first dose of study drug.
The trial enrolled 2,246 patients between July and December 2021 in 20 countries, who were randomized 1:1 to receive either nirmatrelvir/ritonavir or placebo. Enrolment was terminated early, in November 2021, based on positive efficacy results from a planned interim analysis. The median age of the full analysis set (n=2,246) is 46 years (range: 18-88), with 12.8% of patients ≥65 years old. The most common risk factors for COVID-19 critical illness in included patients were obesity (80.5%), cigarette smoking (39.0%), and hypertension (32.9%). 51.2% of participants had baseline positive SARS-CoV-2 antibodies, suggesting current or previous infection.1According to Health Canada regulatory submission documents, among 488 patients who had SARS-CoV-2 isolates sequenced, the Delta variant of concern (VOC) comprised 98.0% of infections.8 94.6% of patients included in the study completed ≥5 days of treatment with study drug. Three patients in the nirmatrelvir/ritonavir group and one patient in the placebo group received monoclonal antibodies for COVID-19 treatment prior to receiving the study intervention.
The primary outcome was a composite of hospitalization >24 hours or all-cause death through Day 28 in the modified intention-to-treat (mITT) population, which included all randomized participants who began treatment ≤3 days after symptom onset and did not receive and were not expected to receive monoclonal antibody therapy.
The key secondary outcome was a composite of hospitalization >24 hours or all-cause death through Day 28 in all randomized patients who began treatment ≤5 days after symptom onset (mITT-1 population). Other secondary outcomes included the incidence of treatment-emergent adverse events by Day 34, incidence of serious adverse events leading to discontinuation of study drug, incidence of adverse events leading to discontinuation of study drug, and viral RNA levels in nasopharyngeal samples through Day 5. Log10-transformed viral loads were compared using an ANCOVA model adjusted for baseline viral load and SARS-CoV-2 baseline seropositivity. Patients with viral loads below the assay’s lower limit of detection (2 log10copies/mL) were imputed as 1.70 log10 copies/mL.
Additional endpoint analyses were undertaken in an additional modified intention-to-treat population (referred to as mITT-2), which included all randomized and treated patients with symptom onset ≤5 days, including those who had received or were expected to receive monoclonal antibody treatment.
Subgroup analyses were predefined in the mITT and mITT-1 populations by age, gender, race, BMI, baseline SARS-CoV-2 serology status, baseline SARS-CoV-2 viral load, baseline comorbidities and number of baseline comorbidities present.
Outcomes
The study population consisting of all those randomized who received at least one dose of intervention within five days of COVID-19 symptom onset and were not expected to receive monoclonal antibody therapy (mITT-1) was considered the most contextually relevant to Ontario because it most closely resembles the patient population in whom nirmatrelvir/ritonavir will be used in clinical practice. Therefore, the mITT-1 population is the basis of our summary of findings unless otherwise stated. The mITT-1 population included 1,039 patients in the intervention group (of the 1,120 randomized) and 1,046 in the placebo group (of the 1,126 randomized).
All sensitivity analyses reported and calculated found similar findings in terms of direction of effect with minor discrepancies in the size of the effect and the confidence interval surrounding the observed findings.1
Composite Outcome of Hospitalization or Mortality
The primary outcome reported in the EPIC-HR study was hospitalization or death by Day 28. There were 8 hospitalizations or deaths in the 1,039 patients that were enrolled in the nirmatrelvir/ritonavir arm, and 66 in the 1,046 patients enrolled in the placebo arm. This represents a statistically significant reduction in hospitalization or death for those who had the intervention (RR 0.12, 95% CI 0.06 – 0.25). This corresponds to a 5.5% absolute risk reduction, meaning one additional hospitalization or death is prevented for approximately every 18 patients treated. However, this is largely driven by the reduction in hospitalizations, as there were 8 hospitalizations in the nirmatrelvir/ritonavir arm and 65 in the placebo arm. Nirmatrelvir/ritonavir significantly reduced hospitalizations compared to placebo (RR 0.12, 95% CI 0.06 – 0.26). This corresponds to a 5.4% absolute risk reduction, meaning one additional hospitalization is avoided for every approximately 18 patients treated.
All-Cause Mortality
At Day 28, there was a statistically significant difference in deaths, with 0 deaths among 1,039 patients in the nirmatrelvir/ritonavir arm and 12 deaths among 1,046 patients in the placebo arm (RR 0.04, 95% CI 0.00 – 0.68). The 1.1% absolute risk reduction means that one death is prevented for every 87 patients treated with nirmatrelvir/ritonavir.
Invasive Mechanical Ventilation
The EPIC-HR trial did not report on this outcome of interest.
Composite Outcome of Invasive Mechanical Ventilation (IMV) or Mortality
Patients who die may not proceed to IMV before death. Therefore, it is important to consider the composite outcome of mortality or progression to IMV. The EPIC-HR trial did not report on this outcome of interest.
Serious Adverse Events
There were 1,109 patients who received nirmatrelvir/ritonavir and 1,115 patients who received placebo analyzed in the safety assessment. There were fewer events in the nirmatrelvir/ritonavir arm, with 18 patients (1.6%) experiencing serious adverse events compared to 74 (6.6%) in the placebo arm (absolute risk reduction 5.0%, 95% CI 3.4 – 6.6%). The most common serious adverse events were COVID-19 pneumonia, worsening of COVID-19, and decreased creatinine clearance in the nirmatrelvir/ritonavir arm, which were not considered to be drug-related by the investigator. Other adverse events included one case of chest discomfort, dyspnea, and palpitations, which resolved at Day 5; one case of facial paralysis, which resolved at Day 37; and one case of abscess with sepsis, which resolved at Day 9. Two patients experienced a grade 4 (life-threatening) adverse event in the nirmatrelvir/ritonavir arm, compared to 10 in the placebo arm. No serious adverse events resulted in death among patients who received nirmatrelvir/ritonavir.
Adverse Events
The EPIC-HR study’s safety evaluation also examined treatment-emergent adverse events. The most frequently reported treatment-related adverse events in the nirmatrelvir/ritonavir group were dysgeusia, diarrhea, D-dimer increases, alanine aminotransferase increase, headache, decreased creatinine clearance, and vomiting. More patients in the treatment arm had dysgeusia (5.6% vs. 0.3%), diarrhea (3.1% vs. 1.6%), headache (1.4% vs. 1.3%), and vomiting (1.1% vs. 0.8%) when compared to placebo. Both dysgeusia and diarrhea were determined to be likely as a result of ritonavir administration. Adverse events resulting in discontinuation of study drug were less common in the nirmatrelvir/ritonavir arm compared to placebo (2.1% vs. 4.2%, respectively). Likewise, treatment-emergent adverse events that were of Grade 3 severity or greater were less common with nirmatrelvir/ritonavir than placebo (4.1% vs. 8.3%).
Outcomes in Patients Who Received Their First Dose between 4 – 5 Days of Symptom Onset
The EPIC-HR study evaluated patients who received their first dose within three days and within five days of symptom onset and findings were robust between these groups. However, the Drugs & Biologics Clinical Practice Guidelines Working Group was interested in the potential impact of starting treatment with nirmatrelvir/ritonavir on days 4 and 5 of symptom onset, given the potential for significant logistical delays in patient access to treatment in Ontario.
Based on the trial data, we calculated there were 342 patients who received their first dose of nirmatrelvir/ritonavir and 364 who received placebo starting on days 4 or 5 of symptom onset. Among these patients, there was no statistically significant difference in mortality, with no events in the nirmatrelvir/ritonavir group and three in the placebo group (RR 0.15, 95% CI 0.01 – 2.93). However, there was a significant reduction in hospitalizations in this population and thus, in the composite outcome of hospitalization and mortality. There were three hospitalizations in the intervention arm, and 21 in the placebo arm (RR 0.15, 95% CI 0.05 – 0.51), corresponding to an absolute risk reduction of 4.9%, or that approximately 20 people would need to be treated to avoid one additional hospitalization. Combined, the findings for the composite outcome were also statistically significant, with 3 events in the nirmatrelvir/ritonavir arm and 22 in the placebo arm (RR 0.15, 95% CI 0.04 – 0.48). This is a 5.1% absolute risk reduction, meaning one event is prevented for every 20 patients treated.
SARS-CoV-2 Viral Load
The EPIC-HR trial evaluated day 5 viral loads in 1,574 included patients, adjusted for baseline viral load, SARS-CoV-2 serological status, and geographic region. Among those patients in whom treatment was initiated ≤5 days from symptom onset, nirmatrelvir/ritonavir decreased SARS-CoV-2 viral load by a mean of -0.69 log10copies/mL (95% CI: -0.86 to -0.53 log10 copies/mL).
Nirmatrelvir/Ritonavir vs. Placebo in Patients with Standard Risk for Progression to Severe Disease
EPIC-SR (NCT05011513) is a phase 2/3 placebo-controlled international study in 20 countries involving both unvaccinated adults at low risk of hospitalization and death and vaccinated adults who had one or more risk factors for progression to severe illness.9 The primary endpoint is self-reported, sustained alleviation of all symptoms for four consecutive days. An interim analysis was posted on December 14, 2021 by the manufacturer.10
Outcomes
The primary outcome, alleviation of symptoms, was not met after 662 patients were recruited and analyzed, but no details are provided.
After 854 patients were analyzed, 0.7% (3/428) of patients receiving nirmatrelvir/ritonavir were hospitalized, compared with 2.3% (10/426) of patients receiving placebo (RR 0.30, 95% CI 0.08 – 1.08), corresponding to a 1.6% absolute risk reduction for hospitalization (95% CI 0.0 to 3.3%), and a number-needed-to-treat of 61 patients to prevent one hospitalization. There were no deaths in either arm.
Full enrollment of EPIC-SR was complete at the time of the press release.
Interpretation
The included studies were multinational randomized placebo-controlled trials designed to assess the effectiveness of nirmatrelvir/ritonavir compared to placebo in people with COVID-19 that are managed in the community and at varying degrees of risk for deterioration. Overall, the trial data for the high-risk population/study were well reported, and several subgroup and sensitivity analyses allowed for assessment of the robustness of the findings. There were concerns with two study sites, however, sensitivity analyses found findings were robust to removal of these sites. Interim analyses demonstrated that the subgroups of those who received treatment within three days of symptom onset were similar to those who received treatment within five days. Our additional calculations demonstrated significant findings for reduced risk of hospitalization when limited to only those who received dosing on days four or five. These findings show preservation of benefit using a timeframe that is more realistic in Ontario with respect to seeking medical care and drug access.
The goal of our recommendations around nirmatrelvir/ritonavir are to apply existing evidence to the Ontario context. The primary outcome of interest is hospitalization; preventing hospitalization is important to patients, and also to the resource-constrained healthcare system. In this study, 6.2% of subjects assigned to placebo were hospitalized. Current Canadian data suggests that patients at highest risk for disease progression (to hospitalization) are those who are older, and increasing age has been the most consistent and important risk factor for hospitalization due to COVID-19.3 However, the median age of individuals enrolled in EPIC-HR was 46 years, and less than 20% of patients were over age 65. This limits the study’s generalizability to Ontarians who are at highest risk of disease progression. Another important risk factor for hospitalization and severe disease in Ontario is being immunocompromised. In a subgroup analysis where patients were stratified by the number of medical comorbidities, there was preservation of effect for those patients with 2-3 comorbidities, which aligns with suggestion from current Canadian data that these patients are at highest risk of poor outcomes.
EPIC-HR did not include anyone who was vaccinated, and included a higher prevalence of people who smoke compared to Ontario (about 30% smokers versus 14% in Ontario).11 However, smoking status did not seem to have significant impact in sensitivity analyses.1 There were also a low number of patients with cardiovascular disease compared to the expected rates in our target population. A genome sequence analysis of 490 participants in Health Canada regulatory submission documents demonstrated that the dominant strain in EPIC-HR was the Delta variant, compared to the current predominance of Omicron in Ontario; this may confer a different hospitalization risk in our target population.12 In addition, a difference in baseline hospitalization rates amongst unvaccinated patients infected with the Omicron VOC compared to the Delta variant may alter the magnitude of the absolute risk reduction for hospitalization; however, no data is available to explore this. There was a theoretical concern that nirmatrelvir may have decreased activity against the Omicron variant due to its ORF1a mutations leading to decreased nirmatrelvir binding to SARS-CoV-2 3CLpro; however, nirmatrelvir was shown to retain in vitro activity against the Alpha, Beta, Gamma, Delta, and Omicron variants of concern.7
Perhaps the most important consideration (addressed below, see “Considerations” section) relates to the absence of information on medication co-administration for study subjects. In the study protocol, subjects who were current or expected users of any medications or substances highly dependent on CYP3A4 for clearance or are strong inducers of CYP3A4 were excluded. We do not know how many patients were enrolled but excluded because of drug-drug interactions. These challenges limit the study’s generalizability to existing Ontario target populations.
Finally, the low event rate of deaths in EPIC-HR is notable; no deaths occurred in the intervention group throughout the study duration. This may result in statistical fragility in the findings, so results for that outcome and should be interpreted with caution. Similarly, the composite outcome of hospitalization or death is not very informative about the impact of nirmatrelvir/ritonavir on deaths, due to the paucity of evidence for this outcome. As such, it should be interpreted more as evidence of effectiveness on the impact of the intervention on hospitalizations rather than true representation of the composite outcome.
Considerations
Drug Interactions
The use of ritonavir in this co-packaged treatment for COVID-19 presents notable challenges with respect to drug-drug interactions which can potentially impact the efficacy of nirmatrelvir/ritonavir, as well as the safety of co-administered medications. A brief summary of significant interactions is provided below; it should be emphasized that 1) the tables are not all-inclusive as it is impossible to include every possible potential interacting medication, and 2) there may be some slight variance from the product monograph based upon pharmacokinetic drug principles, the specific dose and duration of nirmatrelvir/ritonavir therapy, and characteristics of individual medications. This variance from the product monograph also reflects clinical expertise and judgment regarding which interactions are of particular clinical importance. Prescribers are advised to consult the guidance document “Nirmatrelvir/ritonavir: what prescribers and pharmacists need to know”5 and the University of Liverpool COVID-19 Drug Interactions Checker for further details.13 For complex interactions, consultation with a pharmacist experienced in managing ritonavir-related interactions may be helpful.
Drugs Which May Reduce Nirmatrelvir/Ritonavir Concentrations and Negatively Impact Efficacy
Nirmatrelvir and ritonavir are substrates of CYP3A4. Nirmatrelvir/ritonavir is contraindicated in patients taking CYP3A-inducing agents, as these drugs may significantly decrease the concentrations of nirmatrelvir/ritonavir, thus decreasing its efficacy as a COVID-19 treatment (Table 1). Note that this includes current use and recent (i.e., last dose taken within the past 14 days) use of CYP3A-inducing drugs, as dissipation of enzyme induction effects does not occur immediately after drug discontinuation. While offset of enzyme induction may occasionally take up to 4 weeks or longer, data suggest that de-induction generally occurs within two weeks after discontinuation of a potent CYP3A4 inducer. As such, a two-week exclusionary period for enzyme inducers is considered reasonable, particularly as the presence of ritonavir as a booster may help to counteract any lingering enzyme induction effects. Therefore,12-14 any use of enzyme inducers within the past 14 days would preclude the use of nirmatrelvir/ritonavir.

Adapted from: Health Canada. Product Monograph including patient medication information: Paxlovid™. Available at: https://covid-vaccine.canada.ca/info/pdf/paxlovid-pm-en.pdf. Accessed 21 January 2022, and https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-paxlovid-drug-drug-interactions/. Accessed 19 January, 2022.
Drugs Which Are Expected to Be Significantly Increased by Nirmatrelvir/Ritonavir with Risk of Drug Toxicity
Nirmatrelvir/ritonavir is contraindicated in patients taking certain medications that have the potential for serious or life-threatening reactions at high concentrations and are highly dependent on CYP3A4-mediated metabolism (Table 2). Ritonavir acts as a potent inhibitor of CYP3A4, P-gp and other CYP isoenzymes and transporters. The onset of enzyme/transportation inhibition is rapid, and significant: life-threatening inhibition interactions can occur even within the five-day treatment period of nirmatrelvir/ritonavir. With certain drug classes, replacing or interrupting the medication is often difficult due to treatment indication and specificity of pharmacologic effect. In addition, some medications have a prolonged half-life and immediate discontinuation of the drug prior to initiation of nirmatrelvir/ritonavir will not mitigate the interaction risk, particularly since there is a very limited window for initiating nirmatrelvir/ritonavir. Patients on these medications should not receive nirmatrelvir/ritonavir; an alternate COVID-19 therapeutic agent is recommended. If a contraindicated medication can be safely held, this should be done prior to initiating nirmatrelvir/ritonavir.
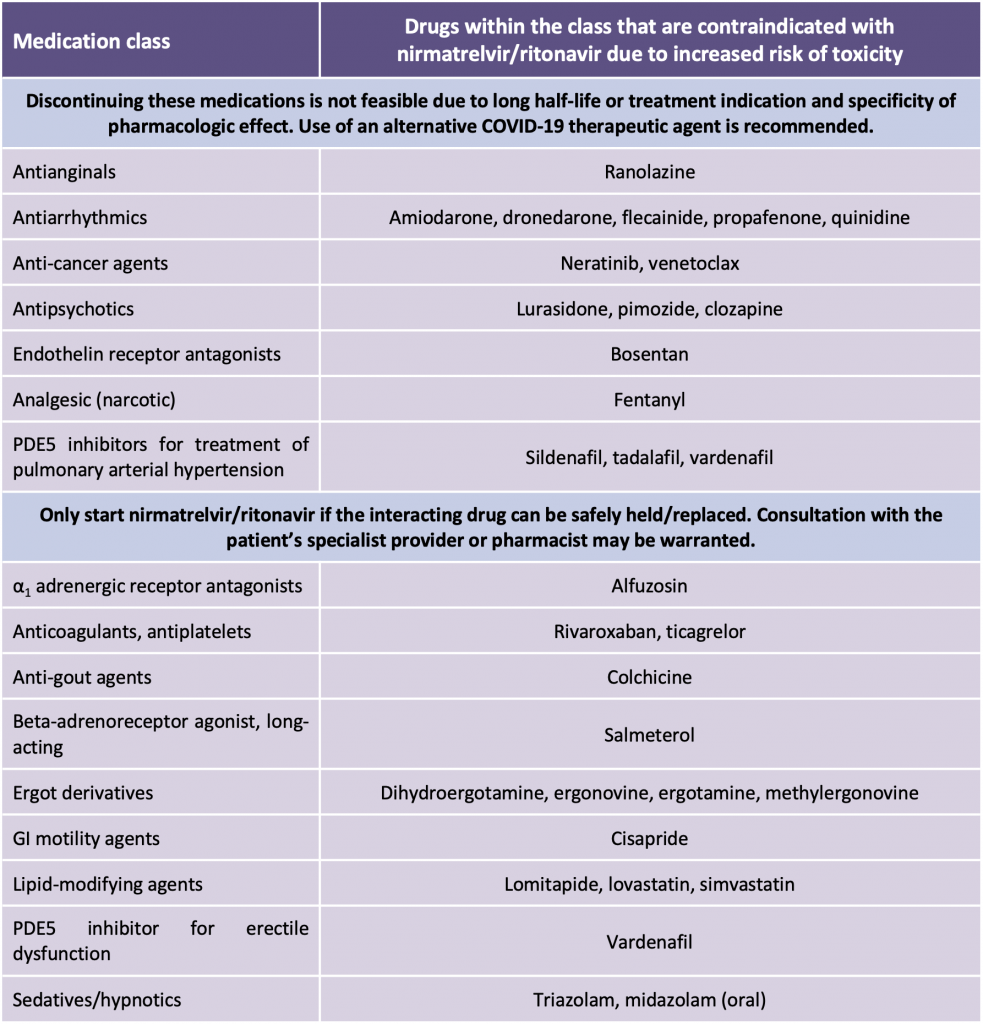
Adapted from: Health Canada. Product Monograph including patient medication information: Paxlovid™. Available at: https://covid-vaccine.canada.ca/info/pdf/paxlovid-pm-en.pdf. Accessed 21 January 2022, and https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-paxlovid-drug-drug-interactions/. Accessed 19 January, 2022.
There are numerous drugs with concentration-dependent toxicities, but where it may be clinically feasible to hold the agent; in some instances, the interaction may be managed by adjusting the dose or dosing interval, or monitoring for increased adverse effects (Table 3). For drugs that have a narrow therapeutic window, such as anticoagulants, antineoplastics, antiplatelets, antiarrhythmics, immunosuppressants, lipid-lowering agents, and psychotropic medications, consultation with a clinical pharmacist/treating specialist is recommended.
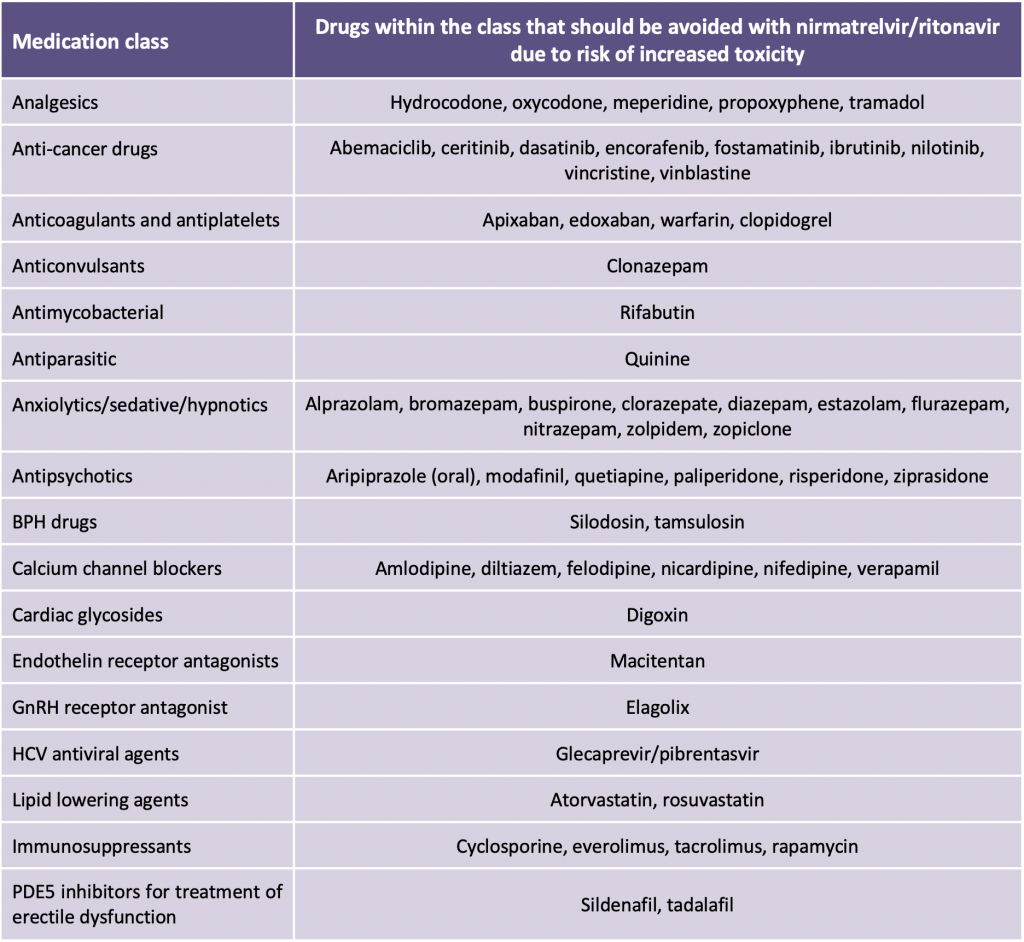
Adapted from: Health Canada. Product Monograph including patient medication information: Paxlovid™. Available at: https://covid-vaccine.canada.ca/info/pdf/paxlovid-pm-en.pdf. Accessed 21 January 2022, and https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-paxlovid-drug-drug-interactions/. Accessed 19 January, 2022.
It should be emphasized that Table 3 does not include all possible drugs which may interact with nirmatrelvir/ritonavir. Notes on interaction management strategies:
Holding Medication
It may take a few days for the offset of ritonavir inhibition effects. Therefore, it is recommended that an interacting medication be held for one week, starting the first day of nirmatrelvir/ritonavir therapy, and resumed two days after completion of therapy unless otherwise specified.
Replacing Medication
Replacing an interacting medication with an alternative agent is generally not a feasible option in the context of nirmatrelvir/ritonavir use, given the short window for treatment initiation. Prescribing a replacement agent may also introduce additional risk if the original drug is not resumed after completion of nirmatrelvir/ritonavirtherapy, or if there is overlapping use of both the replacement agent and original drug. Therefore, when possible, it is preferable to hold an interacting agent.
Dose-Adjustment
Some medications may be continued at a lower dose during nirmatrelvir/ritonavir therapy; expert consultation is recommended as strategies will vary according to the specific medication and indication.
Special Populations – Oncology
It is common practice to hold certain medications such as cytotoxic chemotherapy, some tyrosine kinase inhibitors (TKIs), cyclin-dependent kinase (CDK) inhibitors, and poly (ADP-ribose) polymerase (PARP) inhibitors during acute infections – including COVID-19. Dose adjustment of many chemotherapeutic agents is problematic due to the need for obtaining a new prescription with temporary new dosing instructions, arranging delivery of specialized chemotherapy agents and then resuming therapy at regular doses. For these medications, the preferred course of action is to hold therapy during treatment with nirmatrelvir/ritonavir. Consultation with an oncology prescriber/pharmacist is recommended.
Drugs Which Are Not Expected to Have Clinically Relevant Interactions with Nirmatrelvir/Ritonavir
Ritonavir induces numerous CYP and UGT enzymes; however, maximal onset of induction usually takes up to 2 – 3 weeks to manifest and nirmatrelvir/ritonavir treatment is five days long. High doses of ritonavir can inhibit CYP2D6, but this effect is not clinically relevant with the lower boosting dose used with nirmatrelvir. Some drugs have a wide therapeutic margin and a temporary increase in concentrations is usually well tolerated. Therefore, while the product monograph includes drugs which may interact with nirmatrelvir/ritonavir via these mechanisms, these are not considered to be clinically relevant based on the dose and duration of nirmatrelvir/ritonavir therapy, and characteristics of individual medications. Medications listed in Table 4 may be continued at usual doses during nirmatrelvir/ritonavir therapy.
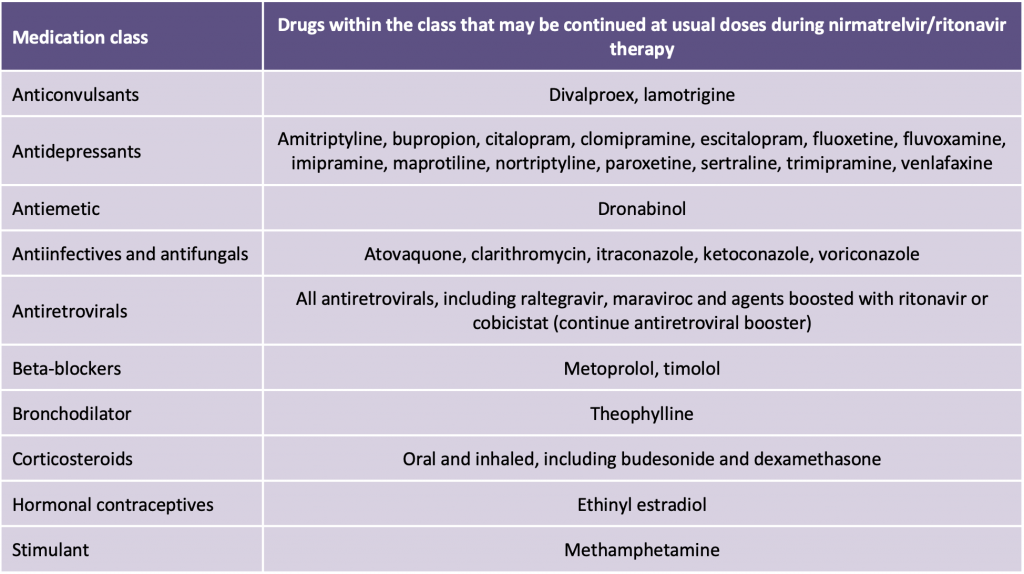
These interactions are not likely to be clinically relevant at the ritonavir dose used and for the limited duration of treatment, as well as the large therapeutic margin of the comedication.
For people living with HIV on antiretroviral therapy: It is recommended that patients continue their antiretroviral therapy at usual dosing while on nirmatrelvir/ritonavir, including those on ritonavir- or cobicistat-containing regimens. Concentrations of some antiretrovirals may be temporarily increased but this is not expected to cause significant issues given the limited duration of nirmatrelvir/ritonavir therapy and tolerability of modern antiretroviral agents. nirmatrelvir/ritonavir will not negatively impact efficacy of antiretroviral treatment or vice versa. Dose adjustments of antiretroviral therapy and nirmatrelvir/ritonavir are not required during the period of nirmatrelvir/ritonavir treatment.
It is strongly recommended to obtain a complete history of medication from all of the patient’s pharmacies including prescription and non-prescription products, natural health products and recreational drug use prior to prescribing nirmatrelvir/ritonavir. Consultation with a pharmacist experienced in managing drug interactions is recommended to screen for any medication interactions that may predispose the patient to a serious or life-threatening adverse outcome with the administration of nirmatrelvir/ritonavir.
Missed Dose
In the event of a missed dose of nirmatrelvir/ritonavir within eight hours of the scheduled administration time, it is recommended that the missed dose should be taken as soon as possible and the normal dosing schedule be resumed.
In the event of a missed dose of nirmatrelvir/ritonavir with ≥8 hours elapsed from the scheduled administration time, it is recommended that the patient skip the missed dose and resume the next dose at the regularly scheduled time.
It is not recommended to double the dose of nirmatrelvir/ritonavir in the event of a missed dose.
Pregnant Patients
Nirmatrelvir/ritonavir may be considered for the treatment of pregnant patients with mild COVID-19 who otherwise meet the criteria outlined for mildly ill patients There is a lack of data on nirmatrelvir/ritonavir use in pregnant patients; however, there is extensive experience with ritonavir use in pregnant patients living with HIV. If a pregnant patient fits the risk profile to potentially derive benefit from nirmatrelvir/ritonavir, the risks and benefits of initiating treatment should be discussed with the patient. Care of pregnant patients with COVID-19 should be managed by a multidisciplinary team with suitable expertise in the management of pregnancy.
There is no data on the use of nirmatrelvir/ritonavir in pregnant individuals with COVID-19. Pregnant patients were excluded from the trial data reviewed in this Science Brief. Pre-clinical studies at supratherapeutic drug levels in rabbits demonstrated reduced fetal body weight at a dose ten times the mean clinical exposure of nirmatrelvir in humans. Evidence from pre-clinical studies and clinical experience currently favour the use of COVID-19 treatment such as sotrovimab in pregnancy compared to nirmatrelvir/ritonavir in situations where sotrovimab is as accessible as nirmatrelvir/ritonavir. Shared decision-making between a pregnant patient and their care provider, and a thorough discussion of the risks and benefits of treatment with nirmatrelvir/ritonavir, is advised.
Lactating Patients
Nirmatrelvir/ritonavir 300 mg/100 mg given orally twice daily for five days may be considered for lactating patients with mild COVID-19 who otherwise meet the criteria outlined for mildly ill patients (see “Recommendations” below).
There is a paucity of data on nirmatrelvir/ritonavir use in lactating patients. However, based on studies in HIV, for which ritonavir is also used, it is known that ritonavir may be present in breast milk. If a lactating patient fits the risk profile to potentially derive benefit from nirmatrelvir/ritonavir, the risks and benefits of initiating treatment should be discussed with the patient. We recommend advising the patient not to breastfeed for the duration of treatment and four days afterwards, during which time breast milk should be pumped and discarded.
Patients with Human Immunodeficiency Virus (HIV) Infection
Nirmatrelvir/ritonavir is recommended in patients with HIV infection taking ritonavir- or cobicistat-containing regimens who otherwise fit the criteria for eligible mildly ill patients (see “Recommendations” below). Patients who are taking ritonavir- or cobicistat-containing regimens for the treatment of HIV infection should remain on those regimens without interruption or adjustment while on nirmatrelvir/ritonavir.
Nirmatrelvir/ritonavir is not recommended in patients with inadequately-controlled HIV-1 infection, nor in patients with presumed/suspected undiagnosed HIV-1 infection, due to the theoretical risk of the development of HIV-1 resistance to protease inhibitors while on ritonavir alone. Physicians should incorporate this risk into their risk-benefit analysis when determining choice of therapy for eligible patients with SARS-CoV-2 infection.
Patients with Renal Impairment
Nirmatrelvir/ritonavir is not recommended in patients with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2, or in patients who require dialysis.
Nirmatrelvir/ritonavir is recommended at a reduced dose of 150 mg/100 mg (one 150 mg nirmatrelvir tablet and one 100 mg ritonavir tablet taken together) given orally twice daily for five days in patients with an eGFR of ≥30-59 mL/min/1.73m2 who otherwise fit the criteria for eligible, mildly ill patients (see “Recommendations” below).
Patients with Hepatic Impairment
Nirmatrelvir/ritonavir is not recommended in patients with severe hepatic impairment, defined as Child-Pugh Class C.
Nirmatrelvir/ritonavir is recommended at a dose of 300 mg/100 mg (two 150 mg nirmatrelvir tablets and one 100 mg ritonavir tablet taken together) given orally twice daily for five days in patients with Child-Pugh Class A or B cirrhosis who otherwise fit the criteria for eligible, mildly ill patients (see “Recommendations” below).
The recommendation in Child-Pugh Class A or B hepatic impairment is of very low certainty and is based on a small pharmacokinetic study in which four doses of nirmatrelvir/ritonavir 100 mg/100 mg were given to eight patients with moderate hepatic impairment and eight controls. The pharmacokinetic parameters Cmax, AUCinf, Tmax, and t1/2 were similar among these two groups of patients, suggesting nirmatrelvir/ritonavir use in Child-Pugh Class A or B hepatic impairment achieves similar therapeutic drug concentrations as healthy controls. There is no pharmacokinetic or safety data in patients with Child-Pugh Class C hepatic impairment.
Supply Chain Issues
It is recommended that, in times of drug shortage, nirmatrelvir/ritonavir be administered to non-hospitalized individuals across Ontario using a hybrid network that includes, but is not limited to, mobile integrated healthcare services, community paramedicine, and outpatient clinics.
There is potential for high global demand of nirmatrelvir/ritonavir among patients with mild COVID-19 who lack pre-existing immunity to SARS-CoV-2, either because they are unvaccinated or immunocompromised. In the event that periods of limited supply cause widespread use of nirmatrelvir/ritonavir to become challenging, we recommend an ethical, evidence-based approach that has been described in detail by the Working Group in previous guidelines.17,18 These guidelines emphasize the preservation of the standard of care for as many patients as possible, and in circumstances where that is no longer possible, the use of a fair and equitable procedure to choose between which patients are allocated available resources.18
Recommendations
Please see the section below entitled “Methods Used for This Science Brief” for a description of COVID-19 illness severity criteria.
Critically Ill Patients (On High-Flow Oxygen, Mechanical Ventilation, or Extracorporeal Membrane Oxygenation (ECMO))
Nirmatrelvir/ritonavir is not recommended for critically ill patients with COVID-19.
Moderately Ill Patients (On Low-Flow Oxygen)
Nirmatrelvir/ritonavir is not recommended for moderately ill patients with COVID-19.
Mildly Ill Patients (Not Requiring Supplemental Oxygen)
Nirmatrelvir/ritonavir is recommended at a dose of 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet), with all three tablets taken together orally twice daily for five days. Patients must be at higher risk of severe disease from COVID-19 and present within five days of symptom onset.
Risk factors for progression to moderate or critical COVID-19 are outlined in in “Methods used for this Science Brief” below, and include immunocompromised individuals, and individuals whose combination of age, vaccination history, and risk factors put them at increased risk of progression to severe disease.
Other treatment options that may be available to these higher risk patients include sotrovimab, remdesivir, fluvoxamine, and budesonide. Clinicians should consider patient-specific factors including (but not limited to) drug-drug interactions, renal function, duration of COVID-19 symptoms, ability to administer intravenous versus oral drugs, strength of evidence, situational context, and drug supply in decision-making regarding choice of therapy (see Therapeutic Management of Adult Patients with COVID-19 summary for additional details).4
If eligible patients with mild COVID-19 who began treatment with nirmatrelvir/ritonavir progress to moderate COVID-19 during their treatment course, they may complete their treatment course at the discretion of the treating physician.
Implementation Considerations
It is recommended that oral antiviral therapy be administered to non-hospitalized individuals across Ontario using a hybrid network that includes, but is not limited to, mobile integrated healthcare services, community paramedicine, virtual/remote assessment, and outpatient clinics.
Special Populations
Pharmacist consultation is important to mitigate any significant drug-drug interactions (including natural products). Nirmatrelvir/ritonavir is contraindicated in patients taking certain medications that have the potential for serious or life-threatening reactions at high concentrations and are highly dependent on CYP3A4-mediated metabolism; and in patients taking certain medications that are CYP3A-inducing, as these may significantly decrease the concentrations of
Nirmatrelvir/ritonavir, decreasing its efficacy as a COVID-19 treatment. (see Tables 1-4 in the “Considerations” section above, and the guidance document “Nirmatrelvir/ritonavir (Paxlovid): What prescribers and pharmacists need to know”).5 For complex interactions, consultation with a pharmacist experienced in managing ritonavir-related interactions may be helpful.
In patients with moderate renal impairment (eGFR ≥30 to <60 mL/min), the dose should be reduced to 150 mg nirmatrelvir (one 150 mg tablet) and 100 mg ritonavir (one 100 mg tablet) taken together twice daily for five days. Nirmatrelvir/ritonavir is not recommended in patients with severe renal impairment (eGFR <30 mL/min) or who require dialysis.
Nirmatrelvir/ritonavir may be considered for the treatment of pregnant patients with mild COVID-19 who otherwise meet the criteria outlined for mildly ill patients There is a lack of data on nirmatrelvir/ritonavir use in pregnant patients; however, there is extensive experience with ritonavir use in pregnant patients living with HIV. If a pregnant patient fits the risk profile to potentially derive benefit from nirmatrelvir/ritonavir, the risks and benefits of initiating treatment should be discussed with the patient. Care of pregnant patients with COVID-19 should be managed by a multidisciplinary team with suitable expertise in the management of pregnancy.
Nirmatrelvir/ritonavir may be considered for lactating patients with mild COVID-19 who otherwise meet the criteria outlined for mildly ill patients. There is a lack of data on nirmatrelvir/ritonavir use in lactating patients; however, based on studies in HIV, for which ritonavir is also used, it is known that ritonavir may be present in breast milk. If a lactating patient fits the risk profile to potentially derive benefit from nirmatrelvir/ritonavir, the risks and benefits of initiating treatment should be discussed with the patient. We recommend advising the patient not to breastfeed for the duration of treatment and four days afterwards, during which time breast milk should be pumped and discarded.
Methods Used for This Science Brief
We searched PubMed, Google Scholar, the COVID-19 Rapid Evidence Reviews, the Joanna Briggs Institute’s COVID-19 Special Collection, LitCovid in PubMed, the Oxford COVID-19 Evidence Service, the World Health Organization’s Global Literature on Coronavirus Disease, and other COVID-19 specific resources listed by the Guidelines International Network and the McMaster Health Forum. We retrieved reports citing relevant articles through Google Scholar and clinicaltrials.gov, and reviewed references from identified articles for additional studies. We also retrieved drug approval submission documentation from Health Canada, the United States Food and Drug Administration, and the European Medicines Agency. The search was last updated on 16 February 2022.
We conducted our meta-analyses using STATA Release 16.0 software (StataCorp LLC, College Station, TX) using a Mantel-Haenszel fixed-effects model.
*Update: The Ontario Science Table Drugs & Biologics Clinical Practice Guidelines Working Group has reviewed its definitions for COVID-19 disease severity. These definitions have always emphasized disease physiology and ventilatory support, as opposed to the setting in which care is delivered. Going forward, definitions for mild, moderate, and critically-ill disease will focus solely on the need for new oxygen or circulatory support and will not reference hospital admission status. These changes are reflected in the definitions below.
For therapeutic recommendations, we used the following definitions for severity:
Critically Ill
Patients requiring ventilatory and/or circulatory support, including high-flow nasal oxygen, non-invasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Moderately Ill
Patients newly requiring low-flow supplemental oxygen.
Mildly Ill
Patients who do not require new or additional supplemental oxygen from their baseline status.
Further Definitions
Immunocompromised or Immunosuppressed Individuals
Examples of immunocompromised or immunosuppressed individuals include receipt of treatment for solid tumors and hematologic malignancies (including individuals with lymphoid malignancies who are being monitored without active treatment), receipt of solid-organ transplant and taking immunosuppressive therapy, receipt of chimeric antigen receptor (CAR)-T-cell or hematopoietic stem cell transplant (within 2 years of transplantation or taking immunosuppression therapy), moderate or severe primary immunodeficiency (e.g., DiGeorge syndrome, Wiskott-Aldrich syndrome, common variable immunodeficiency, Good’s syndrome, hyper IgE syndrome), advanced or untreated HIV infection, active treatment with high-dose corticosteroids (i.e., ≥20 mg prednisone or equivalent per day when administered for ≥2 weeks), alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents classified as severely immunosuppressive, tumor-necrosis factor (TNF) blockers, and other biologic agents that are immunosuppressive or immunomodulatory. These individuals should have a reasonable expectation for 1-year survival prior to SARS-CoV-2 infection.
Risk Factors
Risk factors include obesity (BMI ≥ 30), diabetes, heart disease, hypertension congestive heart failure. Chronic respiratory disease including cystic fibrosis, cerebral palsy, intellectual disability of any severity, sickle cell disease, moderate or severe kidney disease (eGFR <60 mL/min) and moderate or severe liver disease (e.g., Child Pugh Class B or C cirrhosis). Indigenous persons are also considered to be at increased risk of disease progression. If patients have, in the opinion of a physician, other important risk factors for disease progression beyond this list that merit the use of specific drugs or therapeutics, these should be clearly documented at the time of administration.
References
1. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. Published online February 16, 2022. https://doi.org/10.1056/NEJMoa2118542
2. Rai DK, Yurgelonis I, McMonagle P, et al. Nirmatrelvir, an orally active Mpro inhibitor, is a potent inhibitor of SARS-CoV-2 variants of concern. bioRxiv. Published online January 19, 2022. https://doi.org/10.1101/2022.01.17.476644
3. HSIAR Analytics to Action. COVID-19: Hospitalization risk. Preliminary analysis of cases Dec 14 – Jan 6 (Hospitalizations up to Jan 10).; 2022. https://news.gov.bc.ca/files/1.21.22_COVID_Hospitalizations.pdf
4. Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group, Odutayo A, Allen U, et al. Clinical practice guideline summary: Recommended drugs and biologics in adult patients with COVID-19. Ont COVID-19 Sci Advis Table. 2021;Version 1.0. https://doi.org/10.47326/ocsat.cpg.2021.1.0
5. Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group. Nirmatrelvir/ritonavir (Paxlovid): What prescribers and pharmacists need to know. Ontario COVID-19 Science Advisory Table. https://doi.org/10.47326/ocsat.2022.03.55.1.0
6. Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. Published online November 2, 2021. https://doi.org/10.1126/science.abl4784
7. Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198. https://doi.org/10.1016/j.antiviral.2022.105252
8. Health Canada. Product monograph including patient medication information PaxlovidTM.; 2022. https://covid-vaccine.canada.ca/info/pdf/paxlovid-pm-en.pdf
9. Pfizer. An interventional efficacy and safety, phase 2/3, double-blind, 2 arm study to investigate orally administered PF 07321332/ritonavir compared with placebo in nonhospitalized symptomatic adult participants with COVID-19 who are at low risk of progressing to severe illness. clinicaltrials.gov; 2021. https://clinicaltrials.gov/ct2/show/NCT05011513
10. Pfizer. Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death. Published December 14, 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results
11. Government of Canada SC. Smoking, 2019. Published October 22, 2020. https://www150.statcan.gc.ca/n1/pub/82-625-x/2020001/article/00003-eng.htm
12. European Medicines Agency. Assessment report: Paxlovid.; 2021. https://www.ema.europa.eu/en/documents/referral/paxlovid-pf-07321332-ritonavir-covid-19-article-53-procedure-assessment-report_en.pdf
13. University of Liverpool. COVID-19 drug interactions. Published 2022. https://www.covid19-druginteractions.org/checker
14. Punyawudho B, Cloyd JC, Leppik IE, et al. Characterization of the time course of carbamazepine deinduction by an enzyme turnover model. Clin Pharmacokinet. 2009;48(5):313-320. https://doi.org/10.2165/00003088-200948050-00003
15. Reitman ML, Chu X, Cai X, et al. Rifampin’s acute inhibitory and chronic inductive drug interactions: Experimental and model-based approaches to drug-drug interaction trial design. Clin Pharmacol Ther. 2011;89(2):234-242. https://doi.org/10.1038/clpt.2010.271
16. Imai H, Kotegawa T, Tsutsumi K, et al. The recovery time-course of CYP3A after induction by St John’s wort administration. Br J Clin Pharmacol. 2008;65(5):701-707. https://doi.org/10.1111/j.1365-2125.2008.03120.x
17. Morris AM, Bean S, Bell CM, et al. Strategies to manage tocilizumab shortages during the COVID-19 pandemic. Sci Briefs Ont COVID-19 Sci Advis Table. 2021;2(22). https://doi.org/10.47326/ocsat.2021.02.22.1.0
18. Bailey JJ, Morris AM, Bean S, et al. Evidence-based recommendations on the use of casirivimab + imdevimab, and sotrovimab for adults in Ontario. Sci Briefs Ont COVID-19 Sci Advis Table. 2021;2(45). https://doi.org/10.47326/ocsat.2021.02.45.1.0
Document Information & Citation
Author Contributions: ASK, SV, AMM, and MP conceived the Science Brief. ASK, AT, and SV wrote the first draft of the Science Brief. ASK and SV performed the analyses. All authors revised the Science Brief critically for important intellectual content and approved the final version.
The authors would like to thank all members of the Drugs and Biologics Clinical Practice Guideline Working Group for their contribution to this Science Brief.
Citation: Komorowski AS, Tseng A, Vandersluis S, et al. Evidence-based recommendations on the use of nirmatrelvir/ritonavir (Paxlovid) for adults in Ontario. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2022;3(57). https://doi.org/10.47326/ocsat.2022.03.57.1.0
Author Affiliations: The affiliations of the members of the Ontario COVID-19 Science Advisory Table can be found at https://covid19-sciencetable.ca/.
Declarations of Interest: The declarations of interest of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners can be found at https://covid19-sciencetable.ca/. The declarations of interest of external authors can be found under Additional Resources.
Copyright: 2021 Ontario COVID-19 Science Advisory Table. This is an open access document distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited.
The views and findings expressed in this Science Brief are those of the authors and do not necessarily reflect the views of all of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners.
