Key Message
Ivermectin, an antiparasitic agent, is currently not recommended for prophylaxis or treatment of COVID-19. Inappropriate use of ivermectin for COVID-19 may make it unavailable for patients who could benefit from its use (i.e., patients with serious parasitic infections) and reduce the already limited supply of ivermectin in Canada.
However, patients with COVID-19 who receive immunomodulatory therapies (e.g., corticosteroids including dexamethasone, interleukin-6 inhibitors including tocilizumab) may be at risk of dissemination/hyperinfection syndrome from Strongyloides stercoralis, which can be fatal.
We have developed a strategy to safely manage strongyloidiasis risk and infection in the setting of ivermectin shortage. Patients admitted to hospital with COVID-19 and at high epidemiologic risk for strongyloidiasis should be screened with serology. If a patient’s strongyloides serology is reactive or indeterminate, these patients should receive ivermectin to avoid the potential for parasitic dissemination/hyperinfection.
Lay Summary
There Are No Benefits to Using Ivermectin for the Treatment of COVID-19
There is some evidence from laboratory experiments (not conducted in humans) that it may prevent viruses from attacking cells. However, ivermectin has not been shown to be a helpful treatment in other viral illnesses; therefore, based on available evidence, we do not recommend it as an antiviral treatment for COVID-19 infection of any severity. Using ivermectin inappropriately for any possible antiviral effects for COVID-19 may make it unavailable for patients who could benefit from its use (i.e., patients with serious parasitic infections), and reduce the already limited supply of ivermectin in Canada.
Some Recommended Treatments for COVID-19 May Put Certain Patients at Risk of Worsening Parasitic Infections
Patients with COVID-19 who receive therapies that alter immune system function may require ivermectin for the purposes of treating a pre-existing parasitic infection and to avoid severe complications of worsening parasitic infection.
How We Came to Our Recommendations
Ivermectin is approved by Health Canada for the treatment of certain parasitic infections. We reviewed scientific evidence and national/international guidelines on how to screen and identify patients at risk for parasitic infections. We balanced these recommendations with the known (and expected ongoing) limited Canadian supply of ivermectin.
How Ivermectin Works
Ivermectin is an anti-parasitic medication that can be taken by mouth in tablet form or can be applied to the skin.
Ivermectin has an important role in treating parasitic worm infections in humans and animals. These parasitic infections, including one called strongyloidiasis, are more common in certain low- and middle-income countries and can affect Canadian patients who have visited or migrated from these regions. Severe strongyloidiasis can be fatal if not treated.
Our Recommendation for Screening and Treating Parasitic Infections in COVID-19 Patients
Patients with COVID-19 at increased risk for strongyloidiasis who will receive treatment that alters their immune system and may cause worsening of parasitic infection should be screened for strongyloides infection. If a patient’s strongyloides screening test is reactive or indeterminate, they should receive ivermectin treatment.
Summary
Background
Ivermectin is a drug approved for human use in Canada for the treatment of certain parasitic infections.1 There has been speculation that ivermectin may have antiviral effects in vivo.2 However, current evidence does not show that ivermectin has clinical benefit in the treatment of COVID-19.3 At this time, we do not recommend ivermectin for the prophylaxis or treatment of COVID-19 disease.
One approved indication for oral ivermectin is strongyloidiasis due to Strongyloides stercoralis. Strongyloidiasis ranges in presentation from simple intestinal infection (which may be asymptomatic) to hyperinfection (i.e., acceleration of its life cycle in a human host) and disseminated disease, which has mortality rates approaching 90% if left untreated.4,5Immunosuppression, in particular, corticosteroid use, has been reported to provoke the hyperinfection syndrome in patients with chronic strongyloides infection. Dexamethasone and tocilizumab (two therapies recommended by the Science Table for the treatment of moderately and critically ill patients with COVID-19) are anti-inflammatory agents with immunosuppressive effects.6,7 There is no clear relationship or threshold between dose, duration, or concomitant immunosuppression at which the risk of hyperinfection or disseminated disease may occur.4,5,8
This Science Brief will discuss strongyloides screening recommendations for patients with COVID-19, and empiric strongyloides treatment strategies to preserve limited ivermectin supply while ensuring appropriate treatment for patients with COVID-19 who are at risk for hyperinfection (Figure 1).
Questions
How does one assess and manage strongyloides risk in patients with COVID-19?
What symptoms are suspicious for hyperinfection syndrome of disseminated strongyloidiasis?
When should one seek infectious diseases (ID) or tropical medicine expert consultation?
Findings
Assessing and Managing Strongyloides Risk in Patients With COVID-19
Patients with COVID-19 disease being considered to receive immunosuppressive therapy (e.g., dexamethasone, tocilizumab or sarilumab, or both), should have their risk for progressing to disseminated strongyloides evaluated based on both epidemiologic risk and serologic testing.
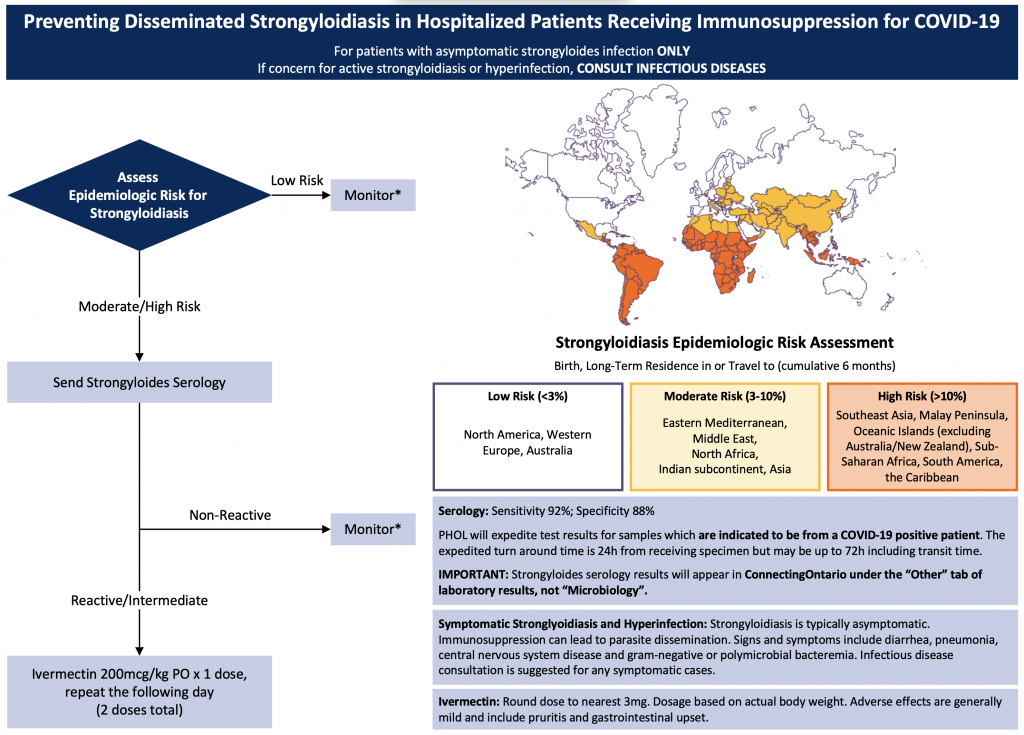
Recommendations are for moderately or critically ill patients.*Monitor for signs and symptoms of strongyloidiasis/hyperinfection.
The prevalence of strongyloidiasis in Canada is not well described, but the Committee to Advise on Tropical Medicine and Travel (CATMAT) estimates that as many as 2.5 million individuals in Canada have simple intestinal strongyloidiasis.4In 2016, CATMAT noted that almost 7 million Canadians are foreign-born, and 85% of this population are from a country where S. stercoralis is endemic. Moreover, in Ontario, the geographic epidemiologic risk factors that place patients at moderate or high risk of strongyloidiasis may be more prevalent in communities that have been disproportionately impacted by the COVID-19 pandemic.9
Step 1 – Epidemiologic Risk Assessment
As outlined in Figure 1, perform an epidemiologic risk assessment based on country of prior residence or extended exposure. “Extended exposure” is generally considered at least 6 months of cumulative residence within a region.4,10Low epidemiologic risk is considered less than 3% risk of infection based on exposure, whereas moderate risk is 3-10%, and high risk is greater than 10%.
Patients at low epidemiologic risk of strongyloidiasis should not have further investigations unless there is clinical suspicion of S. stercoralis hyperinfection (see below). Patients at moderate or high epidemiologic risk of strongyloidiasis should proceed to serological testing.
Note that we assume that the dose of corticosteroid recommended in COVID-19 infection is similar to the CATMAT cutoff for increased risk of hyperinfection and dissemination of “equivalent to 20mg/day of prednisone for ≥ 2 weeks”. Hyperinfection syndrome and disseminated disease has been reported with a range of corticosteroid doses and durations, and it is unknown whether concomitant immunosuppressive therapies contribute to a higher relative risk of hyperinfection and disseminated disease.
Step 2 – Send Strongyloides Serology
Although S. stercoralis testing is ideally performed prior to immunosuppression, this is rarely possible in patients with COVID-19. Patients at moderate or high epidemiologic risk should have Strongyloides serology performed as soon as possible, even if immunosuppression has already been initiated.
In Ontario, STAT Strongyloides serology should be sent to the Public Health Ontario Laboratory (PHOL) indicating that it is from a “COVID-positive patient”. Expected laboratory turnaround time is currently 24 hours, and we recommend waiting for strongyloides serology results before initiating ivermectin, given its limited supply (see “Practical Considerations”).
Although CATMAT guidelines recommend sending both strongyloides serology and stool ova and parasites (OAP), screening with the latter may only result as positive if the patient has a larger burden of infection (e.g., is symptomatic or already experiencing hyperinfection syndrome). Clinicians should note that patients with COVID-19 who also have signs/symptoms of more severe forms of strongyloidiasis will require additional diagnostic testing (4). If there is a strong suspicion of strongyloides hyperinfection at the time of initial COVID-19 presentation (often recognized by unexplained recurrent gram-negative bacteremia, possible pulmonary infiltrates +/- eosinophilia), ID consultation is recommended.
Clinical Findings that are Suspicious for Hyperinfection Syndrome or Disseminated Strongyloidiasis
In patients with impaired immunity, patients with chronic S. stercoralis infection may have “accelerated auto-infection”, known as hyperinfection. They may also have disseminated disease wherein larvae may migrate outside their usual anatomic reservoirs of the gastrointestinal (GI) tract and lungs, resulting in disseminated disease. The migration of the parasite may present as diarrhea, pneumonia, central nervous system infection, or polymicrobial or gram-negative recurrent bacteremia and sepsis. Although chronic strongyloidiasis is often associated with eosinophilia, eosinophilia may not be present once the patient progresses to disseminated disease.
When to Seek ID or Tropical Medicine Expert Consultation
Specialist consultation should be obtained if there is any concern for hyperinfection syndrome with or without dissemination. Clinicians should also consult ID or tropical medicine experts for recommendations on strongyloides treatment in patients from West and Central Africa (areas endemic with Loa loa) due to the risk of severe and possible fatal reactions if ivermectin is administered with concurrent untreated loiasis.4
Practical Considerations
Complications of Ivermectin Use
Ivermectin is generally well tolerated, particularly with the dosing regimens used in the treatment of asymptomatic and mild strongyloides infection. However, occasionally cause skin rash, nausea, vomiting, diarrhea, hepatotoxicity, and neurologic adverse events (including seizures and confusion).1,13
Serologic Testing and Expected Turnaround Time During Pandemic
The recommended test for the purposes of screening for strongyloidiasis (including asymptomatic infection) is S. stercoralis IgG serology, available through Public Health Ontario Laboratory Services (PHOL).14 The usual turnaround time for the result is approximately 7-10 days from receipt by PHOL. Therefore, prior to the pandemic, it was recommended that a pending test result should not preclude empiric ivermectin therapy for patients undergoing immunosuppression who are at very high risk of disseminated strongyloides. During the third wave of the pandemic, PHOL will expediting Strongyloides serology testing if the specimen is clearly marked as coming from a “COVID-19 positive patient”. Strongyloides serology results (as mentioned above) will appear in Lab and Pathology Results of ConnectingOntario under “Other” rather than Microbiology. The estimated turnaround time for expedited testing is 24 hours from receipt by the laboratory. This lab strategy will help to preserve the limited provincial supply of ivermectin, while ensuring that patients who are at the highest risk of progression to hyperinfection are identified as quickly as possible.
Limited Supply of Ivermectin in Canada
Since January 21, 2021, Canadian ivermectin supply has been limited (personal communication, Merck Canada). Therefore, clinicians are encouraged to use ivermectin only when necessary for evidence-based clinical indications, and may need to be prepared to consider using substitute agents if ivermectin is no longer locally available. If substitution is required, consultation with pharmacists and physicians specialized in ID or tropical medicine is recommended.
In a drug shortage scenario, ivermectin should be reserved for the following strongyloides indications, in the following order, based on the “Ethical framework for drug shortages that occur during the COVID-19 pandemic in Ontario”:15
Stage 1
Conserve existing drug supply and consider empiric therapy for very high epidemiologic risk patients (e.g., prior to availability of serology results).
Stage 2
Only treat patients who are asymptomatic for strongyloides with positive serology results and those patients with strongyloides hyperinfection and disseminated disease.
Stage 3
Only treat patients with symptomatic strongyloides infection or highest risk to progress (e.g., critically ill and receiving both dexamethasone and tocilizumab) to strongyloides hyperinfection and disseminated disease.
Recommendations
Please see the section below entitled “Methods Used for this Scientific Brief” for a description of COVID-19 illness severity criteria.
Patients at increased epidemiologic risk for Strongyloides stercoralis infection and admitted to hospital for COVID-19 treatment should have Strongyloides serology performed. If serology is reactive or indeterminate and the patient has asymptomatic strongyloidiasis, they should receive two doses of ivermectin 200 mcg/kg. Patients who have symptomatic strongyloidiasis should receive ivermectin in consultation with an ID physician.
Methods Used for This Science Brief
The literature search for this topic was last updated on April 28, 2021.
For therapeutic recommendations, we used the following definitions for COVID-19 disease severity:
Critically Ill
Patients requiring ventilatory and/or circulatory support, including high-flow nasal oxygen, non-invasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation. These patients are usually managed in an intensive care setting.
Moderately Ill
Patients newly requiring low-flow supplemental oxygen. These patients are usually managed in hospital wards.
Mildly Ill
Patients who do not require new or additional supplemental oxygen from their baseline status, intravenous fluids, or other physiological support. These patients are usually managed in an ambulatory/outpatient setting.
Author Contributions
EL wrote the first draft of the Science Brief. EL, NA, CG, BL, MP, SR, and AMM contributed to the conception of the Science Brief. All authors revised it critically for important intellectual content and approved the final version.
References
1. Merck Canada. Stromectol (Ivermectin) Product Monograph.; 2018. https://www.merck.ca/static/pdf/STROMECTOL-PM_E.pdf
2. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. https://doi.org/10.1016/j.antiviral.2020.104787
3. Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. https://doi.org/10.1136/bmj.m2980
4. Boggild AK, Libman M, Greenaway C, McCarthy AE. CATMAT statement on disseminated strongyloidiasis: Prevention, assessment and management guidelines. Can Commun Dis Rep. 2016;42(1):12-19. https://doi.org/10.14745/ccdr.v42i01a03
5. Stauffer WM, Alpern JD, Walker PF. COVID-19 and dexamethasone: A potential strategy to avoid steroid-related Strongyloides hyperinfection. JAMA. 2020;324(7):623. https://doi.org/10.1001/jama.2020.13170
6. Jüni P, Odutayo A, Allen U, et al. Dexamethasone for Patients Hospitalized with COVID-19.; 2020. https://doi.org/10.47326/ocsat.2020.01.01.1.0
7. Morris AM, Stall NM, Bobos P, et al. Tocilizumab for Hospitalized Patients with COVID-19.; 2021. https://doi.org/10.47326/ocsat.2021.02.11.1.0
8. Winthrop KL, Mariette X, Silva JT, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect. 2018;24:S21-S40. https://doi.org/10.1016/j.cmi.2018.02.002
9. A Strategy for the Mass Distribution of COVID-19 Vaccines in Ontario Based on Age and Neighbourhood – Ontario COVID-19 Science Advisory Table. https://doi.org/10.47326/ocsat.2021.02.10.1.0
10. Buonfrate D, Bisanzio D, Giorli G, et al. The Global Prevalence of Strongyloides stercoralis Infection. Pathogens. 2020;9(6). https://doi.org/10.3390/pathogens9060468
11. Dong MD, Karsenti N, Lau R, et al. Strongyloidiasis in Ontario: Performance of diagnostic tests over a 14-month period. Travel Med Infect Dis. 2016;14(6):625-629. https://doi.org/10.1016/j.tmaid.2016.10.011
12. Buonfrate D, Salas-Coronas J, Muñoz J, et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): a multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect Dis. 2019;19(11):1181-1190. https://doi.org/10.1016/S1473-3099(19)30289-0
13. Chandler RE. Serious Neurological Adverse Events after Ivermectin-Do They Occur beyond the Indication of Onchocerciasis? Am J Trop Med Hyg. 2018;98(2):382-388. https://doi.org/10.4269/ajtmh.17-0042
14. Strongyloides Serology. Public Health Ontario – Laboratory Services. https://www.publichealthontario.ca/en/laboratory-services/test-information-index/strongyloides-serolog
15. Bean S, Denburg A, Greenberg R, Anderson J. Ethical Framework for Drug Shortages That Occur during the COVID-19 Pandemic in Ontario. Joint Centre for Bioethics, University of Toronto; 2020. https://jcb.utoronto.ca/wp-content/uploads/2021/04/Ethical-Framework-for-Drug-Shortages-during-COVID-Pandemic.pdf
Document Information & Citation
Citation: Leung E, McIntyre M, Andany N, et al. Ivermectin as treatment to prevent disseminated strongyloides infection in patients with COVID-19. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;2(30). https://doi.org/10.47326/ocsat.2021.02.30.1.0
Author Affiliations: The affiliations of the members of the Ontario COVID-19 Science Advisory Table can be found at https://covid19-sciencetable.ca/.
Declarations of Interest: The declarations of interest of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners can be found at https://covid19-sciencetable.ca/. The declarations of interest of external authors can be found under Additional Resources.
Copyright: 2021 Ontario COVID-19 Science Advisory Table. This is an open access document distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited.
The views and findings expressed in this Science Brief are those of the authors and do not necessarily reflect the views of all of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners.
