Key Message
Public health agencies have raised concern over cases of acute severe hepatitis of unknown etiology in children that have been reported worldwide. Surveillance has been implemented in several jurisdictions to identify cases, investigate etiologies and monitor trends to determine if there is a signal of concern. The relationship between the COVID-19 pandemic and the genesis of these reports is yet to be fully determined. Potential etiological hypotheses have included adenovirus and SARS-CoV-2 infection. However, to date, cases reported in the published literature have had inconsistent and incomplete testing sent, limiting the epidemiological investigation.
* Routine practices and Additional Precautions in All Health Care Settings, 3rd edition.2 Available at: https://www.publichealthontario.ca/en/health-topics/infection-prevention-control/routine-practices-additional-precautions
** The three pediatric transplant centers are The Hospital for Sick Children (Toronto, Ontario), Stollery Children’s Hospital (Edmonton, Alberta) and CHU Sainte-Justine (Montreal, Quebec).
# See Section on “What tests should be completed” and https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/Hepatitis-of-Unknown-Origin-in-Children
$ https://health.gov.on.ca/en/pro/programs/publichealth/acute_hepatitis/docs/CMOH_order_acute_hepatitis_2022_05_03.pdf.
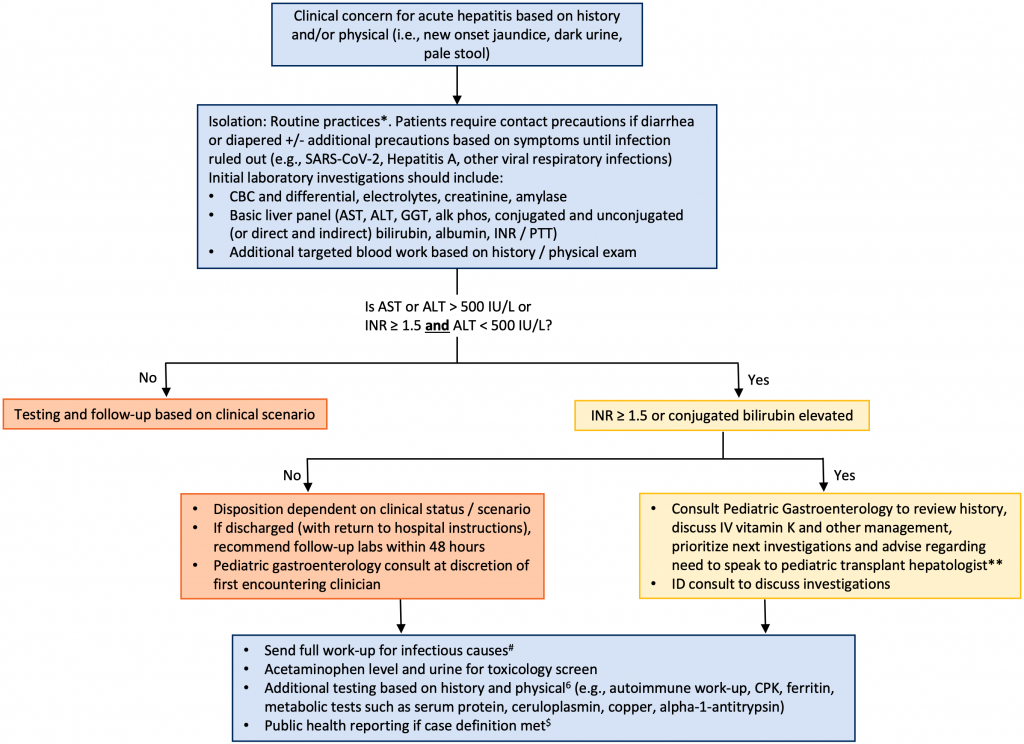
Clinicians need to be aware of how to recognize severity of acute hepatitis in children, what investigations to perform, and threshold to refer to a pediatric gastroenterologist or a liver transplant center. This document summarizes a pathway for the evaluation of children with severe acute hepatitis of unknown etiology and highlights the importance of immediately consulting with a pediatric gastroenterologist if the INR is elevated (greater or equal to than 1.5) and/or serum direct bilirubin is elevated to prioritize investigations and guide management.
Summary
Background
A series of reports originating in Alabama, United States and Scotland in spring 2022 identified a potential concern about an increase in cases of acute severe hepatitis of unknown etiology in children.
Surveillance has been implemented across many jurisdictions globally to identify cases, investigate etiologies, and monitor trends. Given that these reports were emerging during the COVID-19 pandemic, a potential relationship between SARS-CoV-2 infection and acute severe hepatitis is important to explore, among other etiologies. However, many patients have not had complete workups reported in the literature, limiting the epidemiological investigation to date.
Questions
How is severe acute hepatitis of unknown etiology in children defined?
What are potential causes of severe acute hepatitis of unknown etiology in children?
What symptoms should make you suspect acute hepatitis in children?
What tests need to be prioritized and should be completed?
When should I refer?
Findings
Acute severe hepatitis is the sudden onset of liver inflammation and the most severe condition in the spectrum of severe acute hepatitis is pediatric acute liver failure (PALF). There is a broad differential diagnoses for severe acute hepatitis and PALF and it is important to ensure a thorough workup is sent and treatable conditions are recognized early. It is essential that clinicians are able to recognize severe acute hepatitis, decide which diagnostic tests to initiate and when to refer to a pediatric liver specialist or center.
The presence of scleral icterus (yellow pigmentation of the white areas of the eye) or other signs of jaundice are more specific manifestations of severe hepatitis and symptoms that warrant urgent testing. Other less specific symptoms include dark urine and/or pale stools, skin irritation, easy bleeding/bruising, muscle aches, lethargic behaviour, loss of appetite, nausea and vomiting and fever. Recognition of these symptoms combined with a detailed medical history and followed by a combination of clinical, biochemical, radiological and histopathology studies can confirm a clinical diagnosis of acute severe hepatitis.
To date, there is insufficient data to determine whether there has been a recent increase in the incidence of acute severe hepatitis in children. Furthermore, current surveillance data has not conclusively identified a specific infectious or non-infectious cause. A potential role for SARS-CoV-2 or adenovirus infection has been raised but remains unproven at the present time. Several other hypotheses being explored include drug, toxin or environmental exposure or any combination of factors. There is no evidence of a link between any SARS-CoV-2 vaccine with severe acute hepatitis in children, and the majority of cases to date have been among children less than 5 years of age and who were not yet vaccine eligible or who had not received a SARS-CoV-2 vaccine.
Interpretation
The majority of children with acute hepatitis fully recover with supportive care, but the clinical course can be dynamic and can rarely progress to acute liver failure. Clinicians need to be aware of how to recognize severity of acute hepatitis in children, what investigations to initiate, and threshold to refer to a gastroenterologist or liver transplant center. More data is needed to explore a potential relationship between SARS-CoV-2 and adenovirus and acute severe hepatitis in children.
Full Text
Background
Public health agencies have raised concern about a possible increase in severe acute hepatitis of unknown etiology in children. This was initially triggered by reports of 13 cases presenting in Scotland in March 20221and 9 cases from Alabama identified between October 1, 2021 and February 1, 2022.2 Since this time, surveillance has been initiated in many jurisdictions and as of May 27, 2022, the World Health Organization (WHO) reported 650 cases of acute hepatitis of unknown etiology from 33 countries (range < 5 to 222 cases per country) between April 5 and May 25, 2022.3 The challenge is that the baseline incidence of this condition is unknown and the incidence of severe acute hepatitis with the same definitions in prior comparable periods is not available in most jurisdictions. It is therefore difficult to determine whether there has been a true increase in incidence or whether heightened awareness and associated surveillance have led to the increased case identification. In a recent survey, 5 out of 17 European countries and 1 out of 7 non-European countries reported an increase compared with previous years, although limited details were provided on whether and how the numbers were substantiated.4 Notably, recent data from the US did not find an increase in hepatitis-associated emergency department visits, hospitalizations or liver transplants compared to pre-COVID-19 pandemic baseline levels.5
In addition, the incidence of pediatric acute liver failure (PALF; also referred to as fulminant liver failure or fulminant hepatitis), the most severe phenotype of severe acute hepatitis in children, remains uncertain, despite the availability of a clear definition.6 INR values, the key indicator of liver synthetic function used to assess for PALF, were not provided in either of the 2 initial reports,1,2 although data are emerging in more recent reports from transplant centers.7
Given these gaps in the literature, it remains challenging to determine if the current cluster of cases represents a new increased signal of concern. Corresponding cases of severe hepatitis of unknown etiology have not been noted in adults. Nevertheless, a potential increased incidence of severe hepatitis and acute liver failure (as described below) warrants immediate attention and public health agencies worldwide have mandated clinicians to report cases to enable the goal of investigating possible etiologies and monitoring trends. Given the occurrence of these cases during the COVID-19 pandemic, it is important to assess for any possible relationship (or lack thereof) to the pandemic as covered in this document. In this regard, beyond the possibility of a specific new etiologic agent, it is necessary to explore if the pandemic influenced disease epidemiology or reporting patterns culminating in the genesis of the above reports. This document aims to provide background and guidance to clinicians who first encounter children with acute hepatitis, including recommended investigations to evaluate for possible etiologies (summarized below).8
Questions
How is severe acute hepatitis of unknown etiology in children defined?
What are potential causes of severe acute hepatitis of unknown etiology in children?
What symptoms should make you suspect acute hepatitis in children?
What tests need to be prioritized and should be completed?
When should I refer?
Findings
How is Severe Acute Hepatitis of Unknown Etiology in Children Defined?
Acute hepatitis is diagnosed from a combination of clinical, biochemical, and ideally histopathological data, used to confirm the presence and severity of inflammation of the liver. Most patients are previously healthy without clinically significant medical comorbidities, and present with symptoms (liver-specific like jaundice or abdominal distention, or non-liver-specific like nausea, diarrhea, abdominal pain, malaise, etc.) that lead to a clinician ordering bloodwork noteworthy for markedly elevated transaminases. Many have a preceding viral prodrome, often gastrointestinal symptoms including vomiting as a prominent feature.
Acute hepatitis presents along a spectrum of severity, ranging from asymptomatic liver enzyme elevation to PALF. PALF is the most severe condition in the spectrum of severe acute hepatitis. The definition of PALF proposed by the NIH-supported Pediatric Acute Liver Failure Study Group (PALFSG) highlighted distinctions from the longstanding definition in adults in that hepatic encephalopathy is not a defining feature.6 Rather, PALF is defined by biochemical evidence of acute liver injury and hepatic-based coagulopathy (uncorrectable INR ≥ 1.5 in the presence of clinical hepatic encephalopathy or INR ≥2.0 not corrected by IV vitamin K in a child without encephalopathy). PALF accounts for approximately 10-15% of pediatric liver transplants performed in Canada and the United States annually.6 Publications from the PALF Study Group reported that more than 50% of patients were categorized as indeterminate (PALF-I), defined as absence of an identifiable etiology.6
To date, of the 650 probable cases of severe acute hepatitis of unknown etiology identified by the WHO, at least 38 (6%) have required transplantation and nine (1%) deaths have been reported.3 Recent data from the US has not found an increase in hepatitis-associated emergency department visits, hospitalizations or liver transplants compared to pre-COVID-19 pandemic baseline levels.5 That said, within the reported number of cases, it is possible that some new etiologies could exist.
It is difficult to gauge the incidence of acute hepatitis in children as the true denominator for elevated serum ALT levels is unknown due to wide variability in the diagnostic and laboratory work up of symptomatic children.
Surveillance Case Definition of Severe Acute Hepatitis of Unknown Etiology in Children
Triggered by the initial cases in Alabama and Scotland, public health agencies developed surveillance case definitions for this entity. As of June 13, 2022, the current probable case definition developed by WHO and used by many international jurisdictions includes a person who is 16 years or younger presenting with acute hepatitis (non-hepatitis virus A-E) AND serum liver transaminase (AST or ALT) level >500 IU/L, since October 1, 2021.3,9 There are several aspects of the case definition that warrant further discussion:
- Surveillance definitions are not meant to be used as diagnostic definitions or for clinical purposes, they are often broad to enable case finding and explore associations. A sensitive (broad) case definition enables case finding, but will capture other etiologies (both known and unknown) – sensitivity is intentionally high, acknowledging lower specificity. The case definition in all jurisdictions excludes certain etiologies known to cause hepatitis (e.g., hepatotropic viruses – hepatitis A-E), but there is variability in the requirements for reporting cases of acute hepatitis caused by known etiologies.
- Direct comparisons of numbers between jurisdictions may not be accurate if different surveillance case definitions are being used. There are two notable differences in surveillance definitions between jurisdictions. These include 1) variation in the definition used for “severity”, which ranges from biochemical markers alone (i.e., ALT > 500 IU/L) to requiring hospitalization and 2) variation with inclusion/exclusion of cases of acute hepatitis attributed to known etiologies (e.g., autoimmune hepatitis) with available targeted medical treatments.
- Robust and accurate surveillance may be challenging to implement in many jurisdictions and data sources may vary. It is not uncommon for pediatric hospitals to see several children with serum AST or ALT values > 500 IU/L each year and as a result, detailed clinical review is required to identify cases both retrospectively (presenting since October 1, 2021) and prospectively. Clinicians may vary in reporting cases of hepatitis associated with other infections known to cause hepatitis (e.g., herpes group viruses) as well as cases that may be attributed to other known causes of hepatitis in children (e.g., autoimmune hepatitis) with established diagnostic criteria. By keeping the definition broad, the intent is to encourage reporting of all cases, even with known etiologies, which will ultimately help in establishing whether cases are rising and what the cause(s) may be.
- Use of a single biochemical marker (i.e., ALT > 500) as an indicator for severity alone is non-specific. PALF patients can have normal ALT attributed to overwhelming hepatocellular necrosis. Inclusion of INR > 2.0 as an alternative criterion is an important consideration to capture patients with PALF who may not have or no longer have an ALT > 500 U/L and would be missed by the definition focusing on AST or ALT alone.
In Ontario, the current surveillance definition of a probable case includes the following: 1) A person who is 16 years and younger presenting with clinical evidence of severe acute hepatitis since October 2021 and requiring hospitalization, AND 2) elevated serum transaminase >500 IU/L (AST or ALT) or INR > 2.0, AND 3) excluding hepatitis caused or attributed to a hepatitis virus (A,B,C,D,E) or a known or expected presentation of a drug or medication; a genetic, congenital, or metabolic condition; an oncologic, vascular, or ischemia-related condition; or an acute worsening of chronic hepatitis.10 However, cases identified as autoimmune hepatitis (AIH) and hemophagocytic lymphohistiocytosis (HLH) are required to be reported.
Understanding that the surveillance definition assists in detecting a potential signal of an increase in the incidence of severe acute hepatitis is critical to interpreting the reported numbers. However, these numbers have not historically been reported, so it is challenging to determine if there is in fact a signal of concern, unless appropriate methodology for case finding is followed and compared to prior corresponding periods of time. All these factors can lead to uncertainty when examining reported cases across the world.
What Are Potential Causes of Severe Acute Hepatitis of Unknown Etiology in Children?
There is a broad differential for acute hepatitis11,12 and PALF13 in children, including infectious as well as non-infectious conditions. However, to date, there is no conclusive evidence attributing cases of severe acute hepatitis of unknown etiology to any one infectious or non-infectious condition. More robust data are needed to understand whether there is truly an increase in acute hepatitis in children and if so, the cause. Potential etiological hypotheses to date include adenovirus and SARS-CoV-2 infection, although data are limited and inconsistent to date.8
Evidence for Adenovirus
Adenovirus has been detected in some, but not all children reported as cases of severe acute hepatitis of unknown etiology. In the UK case series of 163 children, 126 were tested for adenovirus and 72% were positive on any specimen.8 This corresponded to a time when there was evidence of increased adenovirus detection in the community.8 In the Alabama case series, all 9 patients (100%) tested positive for adenovirus by polymerase chain reaction (PCR) on blood samples (initial viral load range = 991–70,680 copies/mL).2However, since these initial reports, several additional countries have reported cases where adenovirus has not been found (e.g., Israel), or found in a smaller proportion of cases (e.g., Spain, Netherlands, Japan).3
It is important to recognize that adenovirus infections are very common in children. There are more than 100 types of adenovirus divided into seven subgroups (A to G).14 Most children have been infected by at least one adenovirus by the age of 5 years and the virus can be detected by PCR in up to 11% of healthy, asymptomatic children from throat samples.15 Adenovirus may be incidentally detected due to persistence or asymptomatic shedding, limiting the utility of PCR detection alone in respiratory, stool or even blood samples, in attributing causality. While detection in the blood may be considered stronger evidence for a role in hepatitis, testing for adenovirus in blood samples using PCR is rarely performed in otherwise healthy children with uncomplicated infection (i.e., gastroenteritis), so the frequency with which adenovirus is found in the blood is unknown. For a hepatitis to be clearly attributed to a viral infection of the liver, evidence of viral inclusions should be seen on liver pathology,16 as has been reported for adenovirus hepatitis.17 Notably, the liver biopsies from patients in the Alabama case series (n=6) did not show viral inclusions, immunohistochemical evidence of adenovirus or viral particles, which raises significant questions around the role of adenovirus infection as a direct cause of this entity. However, an adenovirus-triggered inflammatory or autoimmune process cannot be excluded. Notably, 6 of the Alabama cases tested positive for EBV DNA in blood by quantitative PCR, but all 5 who were tested for EBV IgM were negative, so these cases did not have acute EBV infection (no such resolution using serology is available for adenovirus).
Adenovirus 41, the strain that has been identified as a possible etiology in recent reports,2 is most commonly associated with gastroenteritis, whereas the serotypes known to cause hepatitis (1,2,3,5 and 7) and other non-40/41 serotypes that have broader cell tropism, have not been identified in any cases to date. Patterns of circulating adenovirus in children without clinical hepatitis have not been reported in most series, making it difficult to assess whether the incidence of adenovirus 41 is elevated amongst those presenting with severe acute hepatitis compared to the general pediatric population in these jurisdictions.
Evidence for SARS-CoV-2
The recent increase in infections worldwide due to SARS-CoV-2, particularly the Omicron variant, coincided with reported cases of hepatitis of unknown etiology. For the majority of case series published and cases reported, SARS-CoV-2 has been infrequently isolated at the time of hepatitis presentation. In the UK case series (n=163), of 132 children tested, 24 (18%) tested positive by PCR, including 11 of the 97 cases (11%) from England.8 Similarly, of the 188 cases from Europe tested for SARS-CoV-2, 23 (12.2%) were positive.18However, when one considers both evidence of acute and previous infection, the strength of association may increase. In a preprint from India,19 among 37 cases of hepatitis between April – July 2021, all were either infected with or had prior evidence of SARS-CoV-2 infection. Furthermore, from the European surveillance data, while serology results were only available on 26 cases, 19 (73.1%) were seropositive.18 However, with the high circulating levels of SARS-CoV-2 in the community for over 2 years, it is possible that these findings may be similar to the prevalence of past/current SARS-CoV-2 infection at the population level in children.
There is pathophysiologic evidence to support a potential role for SARS-CoV-2 in causing hepatitis, as the virus has been shown to have an affinity for the liver.20 SARS-CoV-2 can replicate in cholangiocytes (bile duct cells) and rare cases of severe acute hepatitis where viral-like particles have been seen on liver biopsy.21There have been several retrospective reports demonstrating hepatitis or liver impairment during acute SARS-CoV-2 infection in adults,22,23 with liver test abnormalities estimated to occur in approximately 15% of all hospitalized adult patients.24 However, the overall incidence of liver enzyme elevation in all those with SARS-CoV-2 infection is unknown, as most people do not have liver tests performed during acute infection. While less common in children, hepatitis has also been reported in the context of COVID-19.19,25 A preprint report of a nationwide (US) retrospective cohort study from March 11, 2020 to March 11, 2022 of 245,675 children with a history of COVID-19 (≤10 years old, mean age: 6.0 ± 3.1 years),26 found that children with COVID-19 had a higher risk of elevated AST or ALT (hazard ratio [HR]: 2.52; 95% confidence interval [CI]: 2.03–3.12) and bilirubin (HR: 3.35, 95% CI: 2.16–5.18) at six months after infection compared with a propensity-score matched cohort of those with other respiratory virus infections (n= 245,161).
Given that the majority of children have not demonstrated evidence of acute SARS-CoV-2 infection at the time of hepatitis presentation and the lack of evidence of viral infection in the livers that have been biopsied, a post-infectious cause for hepatitis has also been suggested.27 SARS-CoV-2 has been associated with a number of post-infectious sequelae, including post-acute COVID-19 syndrome (PACS) and multisystem inflammatory syndrome (MIS-C).28 Hepatitis has been reported with MIS-C, with one retrospective review reporting a 43% (n=19) prevalence in pediatric patients.29 It is important to note that the hepatitis was mild in the majority of these patients (ALT < 146 in 14 of the 19 patients), and as a result, the majority of patients would not have met the current case definition for severe acute hepatitis of unknown etiology. Furthermore, the findings of hepatitis in MIS-C are inconsistent, with several systematic reviews and meta-analyses of MIS-C in children not mentioning the occurrence of abnormal liver findings or acute liver failure.30–32 Regardless, it has been suggested that acute hepatitis of unknown etiology could be a post-infectious inflammatory phenomenon related to the Omicron variant, with suggestion of a MIS-C-like immunological injury driven by a superantigen from SARS-CoV-2.33,34 The mechanism remains unclear as to why this would be noted more commonly now that MIS-C appears to be occurring less frequently35 and why the inflammation would be concentrated in the liver.
Other Etiologies Considered
Beyond adenovirus and SARS-CoV-2: While adenovirus and SARS-CoV-2 are leading hypotheses, the etiology and pathogenic mechanisms of recent acute hepatitis cases are still under investigation, and there are several other hypotheses that continue to be explored.8 These include drug, toxin or environmental exposure, a novel pathogen either acting alone or as a coinfection, or any combination of factors. One such combination under investigation includes the “priming hypothesis”, suggesting that the severe hepatitis could be a consequence of an adenovirus infection with intestinal tropism in children who have previously been infected by SARS-CoV-2 and carrying viral reservoirs, leading to a more pronounced superantigen-mediated effect.36The many hypotheses still under investigation and incomplete workup of most cases to date (i.e., inconsistent adenovirus and SARS-CoV-2 testing without exclusion of other etiologies), highlight the need for robust surveillance and thorough workup of potential cases.
No link with vaccines: There is no evidence of a link between any SARS-CoV-2 vaccine with this acute hepatitis entity. The majority of cases have been among children who were less than 5 years of age and who were vaccine ineligible or who had not received a SARS-CoV-2 vaccine. Of the 163 cases reported in the UK, none had received a COVID-19 vaccine.
Clinical Recommendations
What Symptoms Should Make You Suspect Hepatitis?
The presence of scleral icterus or other signs of jaundice are more specific manifestations of severe hepatitis and therefore warrant urgent laboratory testing. Dark urine and/or pale stools are also potentially attributable to hepatitis but less well defined and thus less specific. Considering the appropriate clinical context, other non-specific symptoms that may occur with hepatitis include:
- Pruritus
- Easy bleeding / bruising
- Arthralgia / myalgia
- Lethargy / fatigue
- Loss of appetite
- Nausea, vomiting or abdominal pain
- Fever
What are the Key Points to a Clinical History and Physical Examination?
A detailed history is essential. In addition to routine history, the following information should be collected:
- Date of onset of jaundice (may be most easily identified by timing of darkening of urine), change in mental status, easy bruising, vomiting, and fever
- Exposure to contacts with hepatitis
- History of blood transfusions
- History of travel
- Medication history, both prescription and over the counter in the home (acetaminophen use specifically), including all complementary and alternative medications, and feasibility of intentional or accidental ingestion
- History of depression, suicide attempts, risk-taking behaviours
- Family history of Wilson disease, hepatitis, infant death or autoimmune conditions, which might suggest specific diagnoses
- Evidence of developmental disability and/or seizures, which may suggest inherited metabolic disease or neurological/psychiatric symptoms that may suggest Wilson Disease
- Explore feasibility of a chronic liver condition with an acute or first-time presentation of acute hepatitis – clues include pruritus, abdominal distention or growth failure.
In addition to routine physical examination, the patient should be specifically assessed for:
- Growth, development and nutritional status
- Evidence of bruising, bleeding excessively after venipuncture and petechiae to suspect evidence of coagulopathy
- Findings related to liver dysfunction such as jaundice, hepatomegaly alone or with splenomegaly, ascites, peripheral edema
- Evidence for hepatic encephalopathy, recognizing it may be difficult to assess in toddler and younger aged children. Stages of hepatic encephalopathy are defined slightly differently in children under the age of 4 years.37
- Findings suggestive of chronic liver disease include digital clubbing, palmar erythema, cutaneous xanthoma, and prominent abdominal vessels. If present, these suggest the presence of decompensation or complications of a pre-existing chronic liver condition that has not previously been diagnosed or recognized, rather than a true acute hepatitis or true PALF.
What Tests Should Be Completed?
Initial blood work should include general laboratory tests (CBC and differential, blood glucose, electrolytes, urea, creatinine, amylase), tests of liver injury (AST, ALT, alkaline phosphatase, GGT), and tests of liver function (total and conjugated bilirubin, albumin, INR).
If AST or ALT > 500 IU/L or INR ≥ 1.5, the case should be discussed with a pediatric gastroenterologist or liver specialist for further direction based on the clinical scenario.13 Additional immediate investigations should include an acetaminophen level (even if no history of ingestion), urine for toxicology screen and a complete infectious workup (which is outlined in the Public Health Ontario laboratory information sheet)38 as summarized below. Consultation with a pediatric infectious disease specialist or microbiologist may be helpful to guide investigations based on laboratory test availability in the jurisdiction.
- Respiratory Virus Testing (including SARS-CoV-2 and adenovirus): If performing a respiratory multiplex, confirm whether it includes all adenovirus serotypes. If not all serotypes are included, an adenovirus-specific PCR may be completed on the respiratory sample.
- Stool testing for adenovirus and enterovirus: If performing a stool multiplex, ensure multiplex includes all adenovirus serotypes (some only test for the most common serotypes associated with gastroenteritis, i.e., serotypes 40/41)
- Serology: Anti-HAV IgM and IgG, HBsAg, anti-HBs Ab, anti-HBc IgM and IgG, anti-HCV Ab (or HCV RNA if recent exposure), anti-HEV Ab, EBV EA IgG, VCA IgG and IgM, EBNA IgG, monospot, anti-CMV IgG and IgM, Parvovirus IgM and IgG and SARS-CoV-2 antibodies (anti-S and anti-N)
- Blood PCR: adenovirus (routine), herpes group viruses including CMV, EBV, VZV, HSV (depending on clinical context and if treatment is being considered)
Additional workup may include the following:
- Autoimmune hepatitis evaluation: Quantitative immunoglobulins (IgG, IgA, IgM), antinuclear antibody (ANA), smooth muscle antibody (SMA), liver-kidney microsomal antibody (LKM)
- Toxicology Testing: Urine Toxicology screen
- Other: ferritin, creatine kinase (CK), ceruloplasmin, copper, alpha-1-antitrypsin ceruloplasmin
When Should I Refer?
While acute hepatitis often resolves with supportive care, the clinical course is unpredictable, and clinicians should be cognizant of a potential for rapid deterioration. Clinicians should consult a pediatric gastroenterologist if they have concerns and especially when they are seeing a child with ALT > 500 or INR ≥ 1.5, to discuss investigations, interim management strategies, anticipatory guidance, and frequency of next lab work. Prompt transfer to a Children’s Hospital with a liver transplant program should always be discussed if:
- Persistent or continued elevation of serial INR values and bilirubin levels after one IV vitamin K dosage
- Clinical concerns for hepatic encephalopathy (sleepiness, fatigue, altered mental status, movement abnormalities), GI bleeding, decreased urine output.
Interpretation
For the majority of cases, acute hepatitis resolves with supportive care. Clinicians should consult a pediatric gastroenterologist for a child with AST > 500 IU/L or INR ≥ 1.5 to prioritize investigations,9 discuss management (vitamin K), review anticipatory guidance (clinical monitoring, bleeding prevention), and indications for admission.
More data is needed to explore a potential relationship between suspected etiologies (including SARS-CoV-2 and adenovirus infection) and acute severe hepatitis.
Methods Used for This Science Brief
A rapid review of PubMed and Google Scholar, as well as a cited literature search in Web of Science, was completed on May 30, 2022. Reports citing relevant articles and reference lists of identified articles were also reviewed during this time. Keywords used in this review were: acute hepatitis, children, and COVID-19, however, these were tailored for each database. Specific literature describing these topics was identified manually or through key informants by brief authors following these preliminary database searches.
References
1. Marsh K, Tayler R, Pollock L, et al. Investigation into cases of hepatitis of unknown aetiology among young children, Scotland, 1 January 2022 to 12 April 2022. Eurosurveillance. 2022;27(15):2200318. https//doi.org/10.2807/1560-7917.ES.2022.27.15.2200318
2. Baker JM. Acute hepatitis and adenovirus infection among children — Alabama, October 2021–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71. https//doi.org/10.15585/mmwr.mm7118e1
3. Acute hepatitis of unknown aetiology in children – multi-country. World Health Organization. Published May 27, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/DON-389
4. Beek J van, Fraaij PL, Giaquinto C, et al. Case numbers of acute hepatitis of unknown aetiology among children in 24 countries up to 18 April 2022 compared to the previous 5 years. Eurosurveillance. 2022;27(19):2200370. https//doi.org/10.2807/1560-7917.ES.2022.27.19.2200370
5. Kambhampati AK. Trends in acute hepatitis of unspecified etiology and adenovirus stool testing results in children — United States, 2017–2022. MMWR Morb Mortal Wkly Rep. 2022;71. https//doi.org/10.15585/mmwr.mm7124e1
6. Squires RH, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148(5):652-658.e2. https//doi.org/10.1016/j.jpeds.2005.12.051
7. Deep A, Grammatikopoulos T, Heaton N, Verma A, Dhawan A. Outbreak of hepatitis in children: Clinical course of children with acute liver failure admitted to the intensive care unit. Intensive Care Med. Published online June 10, 2022. https//doi.org/10.1007/s00134-022-06765-3
8. UK Health Security Agency. Technical briefing, investigation into acute hepatitis of unknown aetiology in children in England. GOV.UK. https://www.gov.uk/government/publications/acute-hepatitis-technical-briefing
9. Recommendations for adenovirus testing and reporting of children with acute hepatitis of unknown etiology. Published April 21, 2022. https://emergency.cdc.gov/han/2022/han00462.asp
10. Ontario Ministry of Health. Chief Medical Officer of health order. Published May 20, 2022. https://health.gov.on.ca/en/pro/programs/publichealth/acute_hepatitis/docs/CMOH_order_acute_hepatitis_2022_05_03.pdf
11. Newsome PN, Cramb R, Davison SM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67(1):6-19. https//doi.org/10.1136/gutjnl-2017-314924
12. Malakouti M, Kataria A, Ali SK, Schenker S. Elevated liver enzymes in asymptomatic patients – what should i do? J Clin Transl Hepatol. 2017;5(4):394-403. https//doi.org/10.14218/JCTH.2017.00027
13. Squires JE, Alonso EM, Ibrahim SH, et al. North American society for pediatric gastroenterology, hepatology, and nutrition position paper on the diagnosis and management of pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2022;74(1):138-158. https//doi.org/10.1097/MPG.0000000000003268
14. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. Published online July 1, 2014. https//doi.org/https://doi.org/10.1128/CMR.00116-13
15. Song E, Wang H, Kajon AE, et al. Diagnosis of pediatric acute adenovirus infections: Is a positive PCR sufficient? Pediatr Infect Dis J. 2016;35(8):827-834. https//doi.org/10.1097/INF.0000000000001119
16. Schaefer TJ, John S. Acute hepatitis. StatPearls. Published 2022. http://www.ncbi.nlm.nih.gov/books/NBK551570/
17. Schaberg KB, Kambham N, Sibley RK, Higgins JPT. Adenovirus hepatitis: Clinicopathologic analysis of 12 consecutive cases from a single institution. Am J Surg Pathol. 2017;41(6):810-819. https//doi.org/10.1097/PAS.0000000000000834
18. World Health Organization. Joint ECDC-WHO regional office for Europe hepatitis of unknown origin in children surveillance bulletin. Published June 17, 2022. https://cdn.ecdc.europa.eu/novhep-surveillance/
19. Ratho RK, Asati AA, Mishra N, Jain A, Rawat SK. COVID-19 associated hepatitis in children (CAH-C) during the second wave of SARS-CoV-2 infections in central India: Is it a complication or transient phenomenon. medRxiv. Published online May 9, 2022:2021.07.23.21260716. https//doi.org/10.1101/2021.07.23.21260716
20. Wanner N, Andrieux G, Badia-i-Mompel P, et al. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4(3):310-319. https//doi.org/10.1038/s42255-022-00552-6
21. Fiel MI, El Jamal SM, Paniz-Mondolfi A, et al. Findings of hepatic severe acute respiratory syndrome Coronavirus-2 infection. Cell Mol Gastroenterol Hepatol. 2021;11(3):763-770. https//doi.org/10.1016/j.jcmgh.2020.09.015
22. Abdelrahman MM, Abdel-baset AA, Younis MA, Mahmoud MG, Shafik NS. Liver function test abnormalities in COVID-19 patients and factors affecting them – a retrospective study. Clin Exp Hepatol. 2021;7(3):297-304. https//doi.org/10.5114/ceh.2021.109225
23. Bertolini A, van de Peppel IP, Bodewes FAJA, et al. Abnormal liver function tests in patients with COVID-19: Relevance and potential pathogenesis. Hepatology. 2020;72(5):1864-1872. https//doi.org/10.1002/hep.31480
24. Sultan S, Altayar O, Siddique SM, et al. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159(1):320-334.e27. https//doi.org/10.1053/j.gastro.2020.05.001
25. Brisca G, Mallamaci M, Tardini G, et al. SARS-CoV-2 infection may present as acute hepatitis in children. Pediatr Infect Dis J. 2021;40(5):e214. https//doi.org/10.1097/INF.0000000000003098
26. Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Elevated liver enzymes and bilirubin following SARS-CoV-2 infection in children under 10. medRxiv. Published online May 14, 2022:2022.05.10.22274866. https//doi.org/10.1101/2022.05.10.22274866
27. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. Published online December 10, 2020. https//doi.org/10.1056/NEJMoa2034577
28. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28(5):911-923. https//doi.org/10.1038/s41591-022-01810-6
29. Cantor A, Miller J, Zachariah P, DaSilva B, Margolis K, Martinez M. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: A single-center report. Hepatology. 2020;72(5):1522-1527. https//doi.org/10.1002/hep.31526
30. Ruvinsky S, Voto C, Roel M, et al. Multisystem inflammatory syndrome temporally related to COVID-19 in children from Latin America and the Caribbean region: A systematic review with a meta-analysis of data from regional surveillance systems. Front Pediatr. 2022;10. https://www.frontiersin.org/article/10.3389/fped.2022.881765
31. Santos MO, Gonçalves LC, Silva PAN, et al. Multisystem inflammatory syndrome (MIS-C): A systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J Pediatr (Rio J). Published online December 3, 2021. https//doi.org/10.1016/j.jped.2021.08.006
32. Jiang L, Tang K, Irfan O, Li X, Zhang E, Bhutta Z. Epidemiology, clinical features, and outcomes of multisystem inflammatory syndrome in children (MIS-C) and adolescents—a live systematic review and meta-analysis. Curr Pediatr Rep. 2022;10(2):19-30. https//doi.org/10.1007/s40124-022-00264-1
33. Osborn J, Szabo S, Peters AL. Pediatric acute liver failure due to type 2 autoimmune hepatitis associated with SARS-CoV-2 infection: A case report. JPGN Rep. 2022;3(2):e204. https//doi.org/10.1097/PG9.0000000000000204
34. Yonker LM, Gilboa T, Ogata AF, et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021;131(14). https//doi.org/10.1172/JCI149633
35. Levy N, Koppel JH, Kaplan O, et al. Severity and incidence of multisystem inflammatory syndrome in children during 3 SARS-CoV-2 pandemic waves in Israel. JAMA. Published online May 19, 2022. https//doi.org/10.1001/jama.2022.8025
36. Brodin P, Arditi M. Severe acute hepatitis in children: Investigate SARS-CoV-2 superantigens. Lancet Gastroenterol Hepatol. 2022;7(7):594-595. https//doi.org/10.1016/S2468-1253(22)00166-2
37. Ng VL, Li R, Loomes KM, et al. Outcomes of children with and without hepatic encephalopathy from the pediatric acute liver failure study group. J Pediatr Gastroenterol Nutr. 2016;63(3):357-364. https//doi.org/10.1097/MPG.0000000000001178
38. Public Health Ontario. Hepatitis (acute) – unknown origin in children. Public Health Ontario. https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/Hepatitis-of-Unknown-Origin-in-Children
Document Information & Citation
Author Contributions: VN, MS, UA and FR conceived the Science Brief. VN and MS wrote the first draft of the Science Brief. All authors revised the Science Brief critically for important intellectual content and approved the final version.
Citation: Ng V, Science M, Feld J, et al. Severe acute hepatitis in children of unknown etiology. Science Briefs of the Ontario COVID-19 Science Advisory Table. https://doi.org/10.47326/ocsat.2022.03.63.1.0
Author Affiliations: The affiliations of the members of the Ontario COVID-19 Science Advisory Table can be found at https://covid19-sciencetable.ca/.
Declarations of Interest: The declarations of interest of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners can be found at https://covid19-sciencetable.ca/. The declarations of interest of external authors can be found under Additional Resources.
Copyright: 2021 Ontario COVID-19 Science Advisory Table. This is an open access document distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited.
The views and findings expressed in this Science Brief are those of the authors and do not necessarily reflect the views of all of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners.
