OUTDATED
This version is outdated. Please see https://doi.org/10.47326/ocsat.2021.02.21.2.0 for the latest version of this Science Brief.
Key Message
What do we know so far?
The AstraZeneca COVID-19 vaccine appears to be associated with autoimmune thrombosis that mimics heparin-induced thrombocytopenia (HIT). The United Kingdom, European Union, and Scandinavian countries have reported rare cases of cerebral sinus vein thrombosis (CSVT) and thrombocytopenia in patients who received the AstraZeneca COVID-19 vaccine. The majority of affected patients thus far are women under the age of 55 years, and CSVT seems to occur 4 to 20 days after vaccination. The likely mechanism is antibodies that induce massive platelet activation, reducing the platelet count and causing thrombosis.1,2 This phenomenon mimics heparin-induced thrombocytopenia (HIT) yet it does not require heparin as a trigger. It has been named vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). The incidence of VIPIT appears to be between 1 in 125,000 and 1 in 1 million.3
What symptoms should make you suspect VIPIT?
Patients with VIPIT may present with CSVT, or with other arterial or venous clots. Some symptoms make it more likely that a patient has VIPIT: persistent and severe headache, focal neurological symptoms, seizures, or blurred or double vision (suggesting CSVT or arterial stroke); shortness of breath or chest pain (suggesting pulmonary embolism or acute coronary syndrome); abdominal pain (suggesting portal vein thrombosis); or limb swelling, redness, pallor, or coldness (suggesting deep vein thrombosis or acute limb ischemia). VIPIT seems to occur between 4 to 20 days post-vaccination. Symptoms in this time frame should raise the clinical suspicion of VIPIT.
How do I diagnose VIPIT? How do I rule it out?
Figure 1 presents a decision tree for diagnosing and ruling out VIPIT. Clinicians should ask patients about their COVID-19 vaccine history and should draw a complete blood count (CBC). VIPIT is unlikely if symptoms of blood clotting fall out of the 4-to-20-day time frame OR if the platelet count is ≥ 150 x 109/L.3 VIPIT is more likely if symptoms of blood clotting fall in the 4-to-20-day time frame AND the platelet count is < 150 x 109/L.
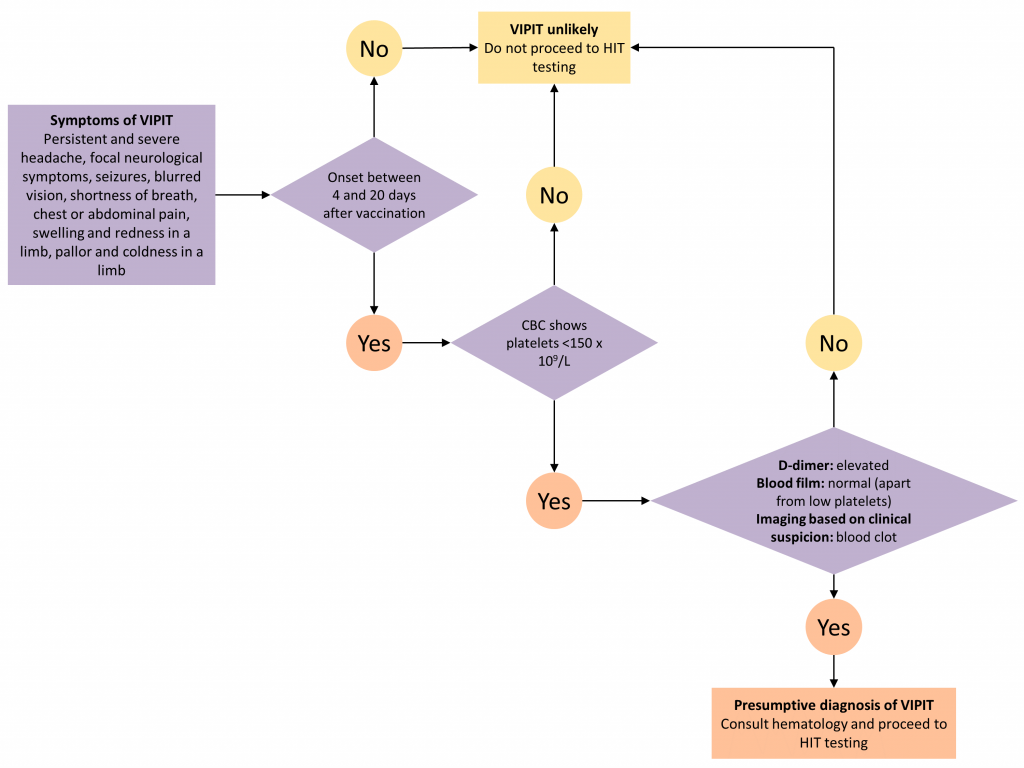
Patients with suspected VIPIT should go on to have a D-dimer level and a blood film drawn. They should also have diagnostic imaging to investigate for blood clots based on clinical suspicion. This should include imaging to rule out CSVT if the patient presents with neurological symptoms, using both parenchymal imaging and vascular imaging, either with a CT head/CT venogram, or MR head/MR venogram. It is not known whether VIPIT, like HIT, is associated with arterial thromboses, but arterial clots should be considered if patients have compatible symptoms. Unenhanced CT brain is a reasonable first diagnostic imaging test if CSVT is suspected, given CSVT’s nonspecific clinical presentation and the importance of ruling out alternate diagnoses. However, the most sensitive diagnostic imaging test for CSVT is an MRI with MR venogram.4 An elevated D-dimer, a normal blood film (apart from thrombocytopenia), and confirmation of a blood clot on diagnostic imaging tests makes the diagnosis of VIPIT presumptive.
The confirmatory diagnosis of VIPIT is made by testing for heparin-induced thrombocytopenia (HIT). This testing should be done even if the patient has had no previous exposure to heparin. HIT testing involves two steps: identification of antibodies against the complex of platelet factor 4 and heparin; and confirmatory functional testing of the antibodies’ ability to activate platelets.5 The HIT enzyme linked immunosorbent assay (ELISA) appears very sensitive to VIPIT; if it is positive, VIPIT is confirmed, and if it is negative, VIPIT is unlikely.2 A number of large hospital laboratories test for the antibodies, but the McMaster University Platelet Immunology Laboratory is the only one lab in Canada performing confirmatory functional testing (testing requisition can be found here). Therefore, presumptive VIPIT should prompt urgent hematology consultation (in person, virtually, or by phone) to arrange for testing and to start safe empiric treatment of blood clots (see below).
How do I treat VIPIT?
The Box presents the treatment principles for patients with presumptive and confirmed VIPIT. Presumptive and confirmed VIPIT should be treated similarly to HIT. Until VIPIT has been ruled out, anticoagulation with heparin (both unfractionated heparin and low molecular weight heparins) should be avoided. Platelet transfusions should not be given.

Alternative anticoagulants that are safe to use in HIT, and likely safe to use in VIPIT, include direct thrombin inhibitors and anti-Xa inhibitors. Most clinicians in Ontario will be comfortable using direct oral anti-Xa inhibitors (e.g., rivaroxaban, apixaban, edoxaban) empirically while awaiting further advice from a hematologist; these agents are used in the treatment of HIT. The dose of direct oral anti-Xa inhibitor is similar to the dose used to treat uncomplicated deep vein thromboses. If the patient has severe renal impairment that makes direct oral anticoagulants unsafe, advice from a hematologist should be sought to guide the use of parenteral anticoagulants that are safe to use in HIT.
How do I treat VIPIT with life threatening blood clots?
In patients with confirmed VIPIT and severe or life-threatening blood clots (e.g., CSVT, splanchnic vein thrombosis), it is important to dampen the prothrombotic response with intravenous immunoglobulin (IVIG). Administration of high dose IVIG (1 g/kg of body weight daily for two days) is appropriate and can be guided by the consulting hematologist.
Is VIPIT a reportable event?
All suspected adverse events following immunization (AEFI), including thrombosis, VIPIT, and suspected VIPIT (which has not been confirmed with HIT testing), should be reported using the provincial AEFI form and sent to the local Public Health Unit. More information on how to report AEFIs can be found on the Public Health Ontario website. Ontario conducts vaccine surveillance safety in collaboration with the Public Health Agency of Canada, and prompt reporting is essential to learn more about this rare but serious thrombotic phenomenon.
References
1. A Prothrombotic Thrombocytopenic Disorder Resembling Heparin-Induced Thrombocytopenia Following Coronavirus-19 Vaccination | Research Square. Accessed March 31, 2021. https://www.researchsquare.com/article/rs-362354/v1
2. Updated GTH statement on vaccination with the AstraZeneca COVID-19 vaccine, as of March 22, 2021. Published March 18, 2021. Accessed March 24, 2021. https://gth-online.org/wp-content/uploads/2021/03/GTH_Stellungnahme_AstraZeneca_3_24_2021.pdf
3. PINHO AC. COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low platelets. European Medicines Agency. Published March 18, 2021. Accessed March 24, 2021. https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots
4. Saposnik G, Barinagarrementeria F, Brown RD, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. https://doi.org/10.1161/STR.0b013e31820a8364
5. Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099-2114. https://doi.org/10.1111/jth.13813
Document Information & Citation
Citation: Pai M, Schull M, Razak F, et al. Vaccine-induced prothrombotic immune thrombocytopenia VIPIT following AstraZeneca COVID-19 vaccination: interim guidance for healthcare professionals in emergency department and inpatient settings. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;1(21). https://doi.org/10.47326/ocsat.2021.02.21.1.0
Author Affiliations: The affiliations of the members of the Ontario COVID-19 Science Advisory Table can be found at https://covid19-sciencetable.ca/.
Declarations of Interest: The declarations of interest of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners can be found at https://covid19-sciencetable.ca/. The declarations of interest of external authors can be found under additional resources.
Copyright: 2021 Ontario COVID-19 Science Advisory Table. This is an open access document distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided that the original work is properly cited.
The views and findings expressed in this Science Brief are those of the authors and do not necessarily reflect the views of all of the members of the Ontario COVID-19 Science Advisory Table, its Working Groups, or its partners.
